
North America Medical Device Coatings Market Research Report: Forecast (2026-2032)
North America Medical Device Coatings Market - By Coating Type (Hydrophilic / Lubricious, Antimicrobial / Infection-Prevention, Drug-Eluting / Drug-Delivery, Anti-Thrombogenic / He...mocompatible, Fluoropolymer / PTFE, Conformal / Barrier, Others), By Substrate (Metals, Ceramics, Polymers, Composites, Glass), By Device Type (Catheters & Guidewires, Stents & Implantables, Surgical Instruments & Tools, Wound Care & Vascular Access, Diagnostics & Electromechanical Devices, Others), By Coating Technology (Dip-Coating, Spray Coating (Ultrasonic / Precision), Vapor Deposition / Parylene (CVD), Plasma / Surface Activation), and others Read more
- Chemicals
- Jan 2026
- Pages 165
- Report Format: PDF, Excel, PPT
North America Medical Device Coatings Market
Projected 6.82% CAGR from 2026 to 2032
Study Period
2026-2032
Market Size (2025)
USD 5.84 Billion
Market Size (2032)
USD 9.27 Billion
Base Year
2025
Projected CAGR
6.82%
Leading Segments
By Device Type: Catheters & Guidewires
North America Medical Device Coatings Market Report Key Takeaways:
- Market size was valued at around USD5.84 billion in 2025 and is projected to reach USD9.27 billion by 2032. The estimated CAGR from 2026 to 2032 is around 6.82%, indicating strong growth.
- By Country, the US holds the largest market share of about 78% in the North America Medical Device Coatings Market in 2025.
- By Coating Type, the Hydrophilic/Lubricious segment represented a significant share of about 32% in the North America Medical Device Coatings Market in 2025.
- By Device Type, the Catheters & Guidewires segment seized a significant share of about 45% in the North America Medical Device Coatings Market in 2025.
- Leading Medical Device Coatings Companies in the North America Market are Surmodics Inc., Biocoat Inc., Hydromer Inc., Harland Medical Systems, Precision Coating Company, Formacoat LLC, AST Products Inc., Covalon Technologies, SCS Coatings, Endura Coatings, Surface Solutions Group, Koninklijke DSM, and Others.
Market Insights & Analysis: North America Medical Device Coatings Market (2026-32):
The North America Medical Device Coatings Market size was valued at around USD5.84 billion in 2025 and is projected to reach USD9.27 billion by 2032. Along with this, the market is estimated to grow at a CAGR of around 6.82% during the forecast period, i.e., 2026-32.
The North America medical device coatings industry outlook is shaped by the integrated medical device manufacturing capacity and cross-border trade flows across the United States, Canada, and Mexico, which create a large base of coated medical products requiring performance-enhancing surfaces. The United States remains the anchor of this ecosystem, serving as the largest single export destination and a core production hub, supported by the United States–Mexico–Canada Agreement (USMCA) that facilitates regional supply chains.
Additionally, in Canada, medical device manufacturing accounted for USD24.4 billion in total output in 2023, with goods exports valued at USD2.7 billion , a segment that includes precision-coated instruments and components integrated into cross-border OEM supply chains. In 2024, the U.S. alone exported USD5.78 billion worth of medical devices to Mexico , showing strong bilateral trade in finished products and supporting the need for consistent, high-performance coatings.
These data points indicate that regional manufacturing scale, deeply integrated cross-border trade, and strong output in core medical device segments collectively sustain robust demand for advanced coatings throughout North America. Regulatory alignment under agencies such as the U.S. FDA and Canada's standards further ensures that coated devices meet safety and performance expectations on both domestic and international fronts.
North America Medical Device Coatings Market Recent Developments:
- 2024 : Biocoat Inc. secured a new patent in August 2024 for its non-PFAS thermal-cure hydrophilic inner-diameter coating method designed for catheter lumens and other medical devices, giving manufacturers a safer PFAS-free option with better lubricity and performance.
- 2026 : Surface Solutions Group announced a long-term investment exceeding USD10 million to build an advanced medical coatings facility in Costa Rica, starting construction early 2026 with operations gradually beginning in 2027 to expand high-precision coating capacity.
North America Medical Device Coatings Market Scope:
| Category | Segments |
|---|---|
| By Coating Type | Hydrophilic / Lubricious, Antimicrobial / Infection-Prevention, Drug-Eluting / Drug-Delivery, Anti-Thrombogenic / Hemocompatible, Fluoropolymer / PTFE, Conformal / Barrier, Others |
| By Substrate | Metals, Ceramics, Polymers, Composites, Glass |
| By Device Type | Catheters & Guidewires, Stents & Implantables, Surgical Instruments & Tools, Wound Care & Vascular Access, Diagnostics & Electromechanical Devices, Others |
| By Coating Technology | Dip-Coating, Spray Coating (Ultrasonic / Precision), Vapor Deposition / Parylene (CVD), Plasma / Surface Activation), and others |
North America Medical Device Coatings Market Driver:
Rising Procedure Volumes and Clinical Performance Requirements
The rapid growth in procedure volumes, combined with higher clinical performance expectations, is driving the market growth, reinforced by regulatory oversight from the U.S. Food and Drug Administration.
For instance, the U.S. performs tens of millions of minimally invasive procedures every year, including cardiovascular, urology, and neurovascular interventions, all of which rely on low-friction, biocompatible coated devices to reduce trauma and procedure time. Similarly, the Centers for Disease Control and Prevention (CDC) estimates that healthcare-associated infections contribute to approximately 1.7 million infections annually in U.S. hospitals , increasing demand for antimicrobial and anti-biofilm coatings on indwelling devices.
Likewise, clinical expectations for longer implant life, reduced thrombosis, and improved deliverability are pushing OEMs to specify coatings as a functional necessity rather than an optional feature. As a result, coatings directly enable safer procedures, better outcomes, and regulatory acceptance, making rising clinical usage intensity and performance standards the single most powerful driver of the North American medical device coatings market.
North America Medical Device Coatings Market Trend:
Nanotechnology-Enabled Functional Coatings Gaining Traction
Nanotechnology-enabled functional coatings are increasingly reshaping the North American medical device coatings landscape by adding biological and performance functions at the nanoscale. These coatings use materials engineered at dimensions typically between 1 nm and 100 nm, which can change surface properties such as antimicrobial action, lubricity, and drug delivery behavior. The U.S. Food and Drug Administration recognizes nanotechnology’s impact and provides guidance for identifying when a regulated product involves nanotechnology, meaning companies must consider nanoscale effects early in development.
Leading coating firms like Specialty Coating Systems (SCS Coatings) are integrating nanostructured antimicrobial parylene through technologies such as microRESIST®, which has demonstrated significant microbial reduction (>5 Log) against common pathogens on coated devices. Other innovators, such as Surface Solutions Group and Hydromer Inc., are exploring nano-enhanced hydrophilic and drug-eluting surfaces that help reduce friction and support localized therapy , reflecting nanotechnology’s integration into functional coatings.
North America Medical Device Coatings Market Challenges:
Regulatory Complexities Hindering Market Growth
Regulatory complexity is the central challenge in the North American medical device coatings market because coatings are not regulated as standalone materials, but as critical components of finished medical devices under the authority of the U.S. Food and Drug Administration. As a result, even minor changes in coating formulation, thickness, curing method, or surface chemistry can force device manufacturers to repeat biocompatibility testing or submit new regulatory filings, directly slowing innovation.
This challenge intensifies because FDA guidance requires coating safety to align with ISO 10993 biological evaluation standards, which mandate multiple toxicological tests, including cytotoxicity, sensitization, irritation, and chemical characterization, before market clearance. Consequently, coating suppliers must generate extensive datasets, even when the coating chemistry is only slightly modified, increasing development costs and timelines.
North America Medical Device Coatings Market (2026-32) Segmentation Analysis:
North America Medical Device Coatings Market Report and Forecast 2026-2032 offers a detailed analysis of the market based on the following segments:
Based on Coating Type:
- Hydrophilic / Lubricious
- Antimicrobial / Infection-Prevention
- Drug-Eluting / Drug-Delivery
- Anti-Thrombogenic / Hemocompatible
- Fluoropolymer / PTFE
- Conformal / Barrier
- Others
Hydrophilic and lubricious coatings dominate this market by holding a market share of about 32% because they directly enable the safe, efficient execution of high-volume minimally invasive procedures, which now form the backbone of modern clinical care. These coatings function by binding water molecules to the device surface, creating a hydrated layer that dramatically lowers friction during insertion and navigation through blood vessels, organs, and tissue pathways. This performance advantage makes them indispensable for catheters, guidewires, introducer sheaths, and delivery systems used daily across cardiovascular, neurovascular, and urological procedures.
Their importance is formally acknowledged by the U.S. Food and Drug Administration, which issues specific guidance on labeling and performance expectations for intravascular devices with lubricious coatings, underscoring that these surfaces are not optional enhancements but critical functional components. Clinically, hydrated hydrophilic coatings can reduce surface friction to extremely low levels, significantly decreasing insertion force, vessel trauma, and procedural time, contributing to their dominance.
Based on Device Type:
- Catheters & Guidewires
- Stents & Implantables
- Surgical Instruments & Tools
- Wound Care & Vascular Access
- Diagnostics & Electromechanical Devices
- Others
Catheters and guidewires dominate the North American Medical Device Coatings Market, accounting for about 45% market share because they are among the most frequently deployed invasive devices and inherently depend on surface performance to ensure safety and functionality. In the United States, over 30 million urinary catheters are used annually, and an estimated 15–25% of hospitalized patients receive at least one urinary catheter during their stay , directly driving large-scale demand for lubricious and antimicrobial coatings. Additionally, U.S. healthcare facilities record approximately 15 million central venous catheter (CVC) days each year, highlighting the extensive use of intravascular catheters that require hemocompatible and low-friction coatings to reduce thrombosis and bloodstream infections (CDC).
Guidewires further reinforce this dominance because they are essential in nearly all cardiovascular, peripheral vascular, and interventional radiology procedures, where smooth navigation and torque control depend on high-performance hydrophilic coatings. Even minor surface improvements can significantly reduce procedure time and vessel trauma, making coatings a functional necessity rather than an enhancement.
North America Medical Device Coatings Market (2026-32): Regional Projection
The United States leads the North American medical device coatings industry largely with a market share of about 78% because it is also the largest global medical device market, representing more than 40 % of global medtech revenues and forming the core demand that drives coatings innovation and capacity . The U.S. medical device industry directly exports over USD103 billion of devices in 2023 , making it a major manufacturing and export hub that attracts suppliers of specialized coatings used on catheters, stents, implants, and other high-performance devices.
Structurally, the U.S. Food and Drug Administration (FDA) regulates all medical device production and coatings used on them, meaning regulatory compliance, safety, and quality standards are set in the U.S. and referenced globally, prompting both domestic and international firms to locate R&D and coating manufacturing near U.S. OEMs. These regulatory drivers amplify demand for advanced coatings like hydrophilic, antimicrobial, and drug-eluting types that meet stringent FDA performance requirements.
Furthermore, the concentration of medtech innovation and manufacturing in the U.S. increases the adoption of advanced coatings, as firms seek performance improvements to maintain competitiveness and satisfy export requirements tied to U.S. regulatory scrutin
Gain a Competitive Edge with Our North America Medical Device Coatings Market Report:
- North America Medical Device Coatings Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size & share, growth rate, competitive landscape, and key players. This comprehensive analysis helps businesses gain a holistic understanding of the market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing businesses to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- North America Medical Device Coatings Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, businesses can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Introduction
- Product Definition
- Research Process
- Assumptions
- Executive Summary
- North America Medical Device Coatings Market Regulations, Policies & Standards
- North America Medical Device Coatings Market Trends & Developments
- North America Medical Device Coatings Market Supply Chain Analysis
- North America Medical Device Coatings Market Imports/Exports
- North America Medical Device Coatings Market Dynamics
- Growth Drivers
- Challenges
- North America Medical Device Coatings Market Hotspots & Opportunities
- North America Medical Device Coatings Market Pricing Analysis
- North America Medical Device Coatings Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- By Volume (Thousand Tons)
- Market Share & Analysis
- By Coating Type- (USD Million & Thousand Tons)
- Hydrophilic / Lubricious
- Antimicrobial / Infection-Prevention
- Drug-Eluting / Drug-Delivery
- Anti-Thrombogenic / Hemocompatible
- Fluoropolymer / PTFE
- Conformal / Barrier
- Others
- By Substrate- (USD Million & Thousand Tons)
- Metals
- Ceramics
- Polymers
- Composites
- Glass
- By Device Type- (USD Million & Thousand Tons)
- Catheters & Guidewires
- Stents & Implantables
- Surgical Instruments & Tools
- Wound Care & Vascular Access
- Diagnostics & Electromechanical Devices
- Others
- By Coating Technology- (USD Million & Thousand Tons)
- Dip-Coating
- Spray Coating (Ultrasonic / Precision)
- Vapor Deposition / Parylene (CVD)
- Plasma / Surface Activation
- By Country
- The US
- Mexico
- Canada
- Rest of North America
- By Competitors
- Competition Characteristics
- Market Share & Analysis
- By Coating Type- (USD Million & Thousand Tons)
- Market Size & Analysis
- The US Medical Device Coatings Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- By Volume (Thousand Tons)
- Market Share & Analysis
- By Coating Type- (USD Million & Thousand Tons)
- By Substrate- (USD Million & Thousand Tons)
- By Device Type- (USD Million & Thousand Tons)
- By Coating Technology- (USD Million & Thousand Tons)
- Market Size & Analysis
- Mexico Medical Device Coatings Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- By Volume (Thousand Tons)
- Market Share & Analysis
- By Coating Type- (USD Million & Thousand Tons)
- By Substrate- (USD Million & Thousand Tons)
- By Device Type- (USD Million & Thousand Tons)
- By Coating Technology- (USD Million & Thousand Tons)
- Market Size & Analysis
- Canada Medical Device Coatings Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- By Volume (Thousand Tons)
- Market Share & Analysis
- By Coating Type- (USD Million & Thousand Tons)
- By Substrate- (USD Million & Thousand Tons)
- By Device Type- (USD Million & Thousand Tons)
- By Coating Technology- (USD Million & Thousand Tons)
- Market Size & Analysis
- North America Medical Device Coatings Market Key Strategic Imperatives for Growth & Success
- Competitive Outlook
- Company Profiles
- Surmodics Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Biocoat Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Hydromer Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Harland Medical Systems
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Precision Coating Company
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Formacoat LLC
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- AST Products Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Covalon Technologies
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- SCS Coatings
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Endura Coatings
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Surface Solutions Group
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Koninklijke DSM
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- Surmodics Inc.
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
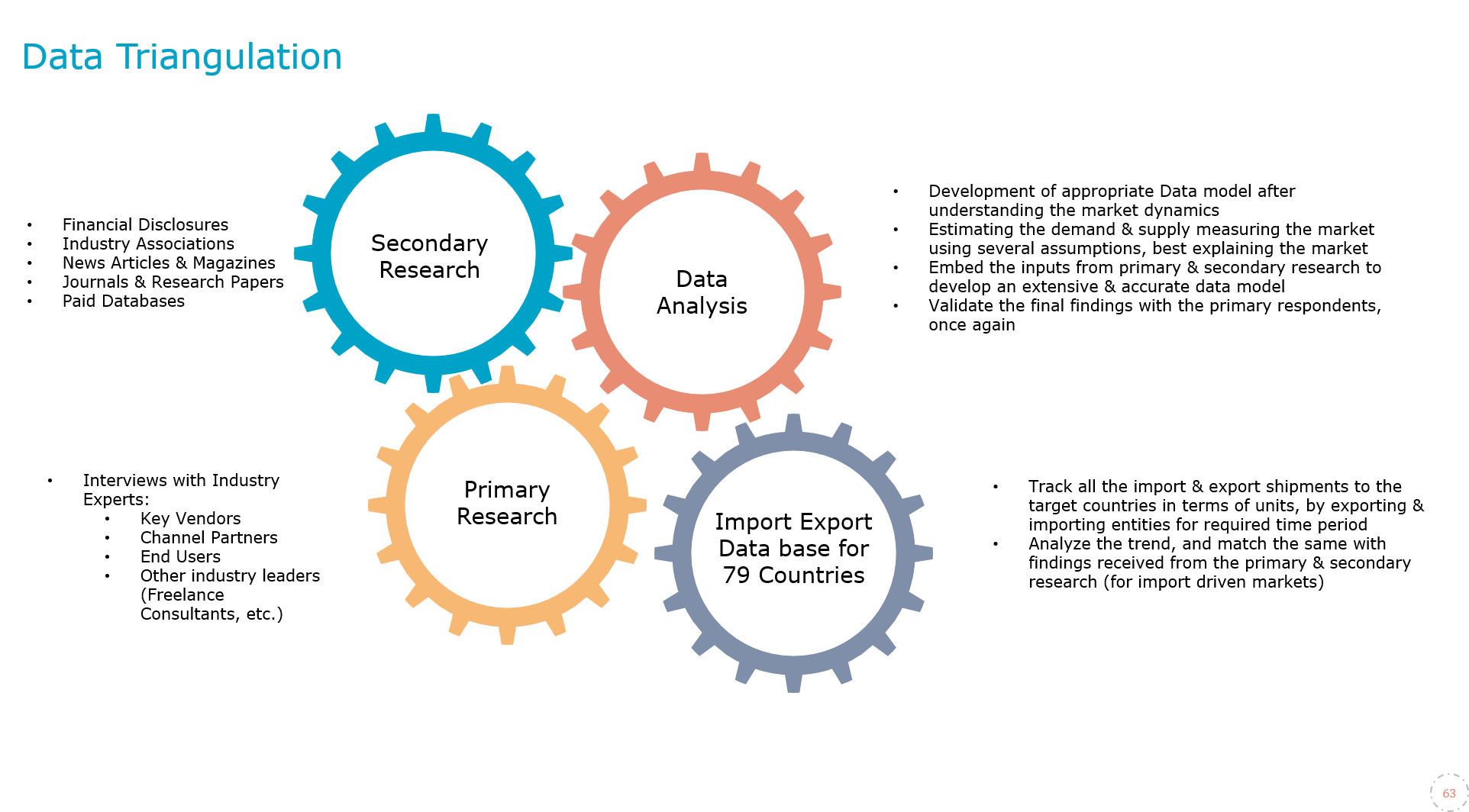
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









