
Australia Reprocessed Medical Devices Market Research Report: Forecast (2025-2030)
Australia Reprocessed Medical Devices Market - By Device Type (Cardiovascular Devices, Laparoscopic Devices, Gastroenterology Devices, General Surgery Devices, Orthopedic Devices, ...Others), By Classification (Critical, Non-Critical, Semi-Critical), By End User (Hospitals, Diagnostic Centers, Ambulatory Surgical Centers) and others Read more
- Healthcare
- Nov 2024
- Pages 117
- Report Format: PDF, Excel, PPT
Market Definition
Reprocessed medical devices are devices that have been used on patients, then accumulated, cleaned, disinfected, tested, and restored to fulfill original equipment manufacturer (OEM) specifications or validated processing requirements. These devices go through stringent quality control processes to ensure they may be secure and effective for reuse. Reprocessing allows healthcare centers to reduce costs, manage medical waste, and keep satisfactory care at the same time as adhering to regulatory requirements for affected person safety.
Market Insights & Analysis: Australia Reprocessed Medical Devices Market (2025-2030):
The Australia Reprocessed Medical Devices Market size was valued at around USD 319 million in 2024 and is projected to reach USD 506 million by 2030. Along with this, the market is estimated to grow at a CAGR of around 8% during the forecast period, i.e., 2025-30. Several crucial elements are driving the growth of the Australia Reprocessed Medical Devices Market. Hospitals and healthcare providers are under economic pressure to reduce costs and that is the main reason for the growing demand for affordable healthcare solutions.
| Report Coverage | Details |
|---|---|
| Historical Years | 2020-23 |
|
Base Years
|
2024
|
|
Forecast Years
|
2025-30
|
| Market Value in 2024 | USD 319 Million |
| Market Value in 2030 | USD 506 Million |
| CAGR (2025-30) | 8% |
| Top Key Players | Stryker, ReNu Medical, Inc., Medline Industries, Cleanpart Gmb, Cardinal Health ANZ, Hygia Healthcare, SureTek Medical, NEScientific, Inc., Medtronic PLC, Johnson & Johnson, and Others |
| Key Report Highlights |
|
*Boost strategic growth with in-depth market analysis - Get a free sample preview today!
Reprocessed clinical devices like surgical devices are less expensive than new devices and mainly useful in an environment in which cost efficiency is paramount. Further, as healthcare organizations plan to reduce their waste and environmental impact, there's an increasing emphasis on sustainability on this subject. Additionally, the use of reprocessed medical devices in Australia has also been significantly aided by the authority's regulations and applications. Before being utilized in medical settings, reprocessed devices must pass stringent safety and effectiveness regulations overseen by organizations such as the Therapeutic Goods Administration (TGA). The technological developments in reprocessing methods and devices allow for extended efficiency and better-preprocessed device quality. These developments attract a wide range of end-users, thus augmenting the size & volume of the industry.
New opportunities are also rising in the market with the growing number of Australian population. Reprocessed devices have a significant market due to the rise in complications related to old age and chronic illnesses, that need medical treatments. Additionally, reprocessed alternatives could benefit rural healthcare settings that often struggle to get expensive new medical devices. Moreover, there is growing potential for strategic partnerships between reprocessing businesses and hospitals that purpose to improve device quality and increase the use of reprocessed products in numerous industries such as outpatient care.

Furthermore, current trends show a huge shift towards environmentally friendly practices in the healthcare industry as more Australian institutions are adopting reprocessed medical devices. In recent times the reprocessing method has been streamlined with the help of artificial intelligence (AI) and other modern technology which enhance device sterilization and tracking. These patterns demonstrate the marketplace's capability for sustained expansion and innovation in the Australia Reprocessed Medical Devices Market.
Australia Reprocessed Medical Devices Market Driver:
Increased Adoption of Sustainable Healthcare Practices – To lessen its environmental impact, the Australian healthcare sector is enforcing sustainable practices. Medical device reprocessing gives a greater sustainable opportunity to the traditional practice of using devices once. Reprocessing reduces medical waste notably by enabling the cleaning, sterilization, and reuse of medical systems along with surgical instruments, endoscopes, and catheters. Both the industry’s carbon footprint and waste disposal costs are reduced through reprocessing. In addition, to decreasing the need for energy and raw materials to manufacture new devices, reprocessing medical devices also allows to reduce cost and effectively aligns with environmental efficiency goals. The use of recycled medical systems is growing as hospitals area more emphasis on eco-friendly procedures that are consistent with the industry’s larger sustainability targets. For Instance,
- In 2024, St. Vincent’s Hospital in Melbourne applied an application to reprocess devices like surgical equipment and catheters to reduce environmental effects, in step with Australia's sustainability goals.
Australia Reprocessed Medical Devices Market Opportunity:
Supportive Government Guidelines Promoting Reprocessed Medical Devices – In Australia, government guidelines have played a significant role in promoting the use of reprocessed medical devices, pushed by the growing awareness of healthcare sustainability and waste reduction. The Australian Authorities Therapeutic Goods Administration (TGA) is answerable for regulating medical devices, which includes reprocessed ones, to ensure they meet stringent safety requirements. The National Health Service (NHS) and other healthcare bodies are also increasingly adopting suggestions that encourage the reprocessing of medical devices to reduce environmental effects.
In 2023, the Australian Commission on Safety and Quality in Health Care (ACSQHC) highlighted the importance of environmentally responsible practices inside healthcare, encouraging hospitals and healthcare centers to adopt reprocessing techniques wherein suitable. Regulatory frameworks have been updated to make sure that reprocessed devices, like endoscopes and catheters, are safe and meet the best standards. This regulatory push continues to create opportunities for the growth of the reprocessed clinical devices market in Australia.
Australia Reprocessed Medical Devices Market Challenge:
High Risks Associated with Reprocessed Device Performance – The major challenge in the Australian Reprocessed Medical Devices Market is dealing with the risks concerned with the operation of reprocessed gadgets preserving patient protection. Although reprocessed medical devices can reduce costs and waste, they can pose the hazard of failing if not performed to the best standards. Insufficient sterilization poses the main risk as it can lead to transmission of infections and reduce the device's capability with repeated use. Also, errors, blockages, or mechanical failures that compromise patient safety during medical procedures can arise from improperly reprocessed devices. Especially the risk of wear and tear during reprocessing devices like endoscopes, catheters, and surgical contraptions. Ineffective control may also lessen the device’s efficacy and increase the risk of damage throughout medical procedures.
Australia Reprocessed Medical Devices Market Trend:
Technological Advancements in Cleaning & Sterilization with AI/Robotics Integration – The incorporation of technological developments in cleaning and sterilization, with the application of artificial intelligence and robotics, is a noteworthy trend in the Australian Reprocessed Medical Devices Market. The need for uniformity effectiveness and safety in clinical settings is using automatic systems for cleaning and sterilizing medical devices. The use of technologies like ultraviolet (UV) sterilization and robotic cleaning systems is gaining popularity as approaches to improve cleaning lower human errors and assure a higher degree of sterilization.
AI is essential to tracking and improving these procedures. Predictive analytics powered by AI can assist in tracking equipment performance, identifying issues, and forecasting maintenance requirements ensuring that equipment is properly cleaned and sterilized. Medical device reprocessing is becoming faster and more accurate with robotics and artificial intelligence (AI) enhancing process automation. For instance;
- Steelco disassembles, cleans, and reassembles complex devices, automated with robotic arms lowering the possibility of contamination and human error.
Australia Reprocessed Medical Devices Market (2025-2030): Segmentation Analysis
The Australia Reprocessed Medical Devices Market study of MarkNtel Advisors evaluates & highlights the major trends and influencing factors in each segment. It includes predictions for the period 2025–2030 at the global level. Based on the analysis, the market has been further classified as:
Based on Device Type:
- Cardiovascular Devices
- Laparoscopic Devices
- Gastroenterology Devices
- General Surgery Devices
- Orthopedic Devices
- Others
Cardiovascular devices are leading the Australia Reprocessed Medical Devices Market accounting for 40% of the entire marketplace. The dominance is due to a lot of cardiovascular procedures being completed each year thus reprocessing those devices can be cost savings. Due to their essential role in patient care devices used in cardiology treatment like stents, catheters, and guidewires are often subject to strict regulations. For the increasing demand for cardiovascular treatments in Australia's aging population, reprocessing presents a cost-effective alternative as these gadgets are normally single-use. Upgradation in cleansing and sterilization techniques and reprocessed cardiovascular devices are being adopted as they ensure safety standards while maintaining performance. Also, the high price of new cardiovascular devices encourages healthcare providers to undertake reprocessing devices to decrease overall healthcare costs.
Based on End User:
- Hospitals
- Diagnostic Centers
- Ambulatory Surgical Centers
The hospital segment is leading by capturing a 60% share of the Australian Reprocessed Medical Devices Market due to the high volume of medical procedures and the substantial cost-saving opportunity. Because of the frequent use in patient care, hospitals regularly need multiple medical devices which include endoscopes, catheters, and surgical contraptions. Reprocessing devices which are typically single-use, offer significant economic benefits by reducing the need to buy new equipment. Additionally, hospitals are under pressure to enforce sustainable practices and reduce waste which aligns with Australia’s growing emphasis on eco-friendly healthcare. Each of those issues is addressed by using reprocessed devices that are cost-effective and eco-friendly alternatives. Due to these factors, the hospital end-user dominates Australia’s market for reprocessed medical devices.
Australia Reprocessed Medical Devices Market Recent Development:
- July 2024: Cardinal Health ANZ introduced a new facility for reprocessing single-use medical devices which will strengthen Australia’s sovereign healthcare capabilities, create jobs, improve healthcare supply resilience, and reduce waste.
Gain a Competitive Edge with Our Australia Reprocessed Medical Devices Market Report
- Australia Reprocessed Medical Devices Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size, growth rate, competitive landscape, and key players. This comprehensive analysis helps business organizations to gain a holistic understanding of market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing business organizations to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- Australia Reprocessed Medical Devices Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, business organizations can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Research Methodology
- Product Definition
- Research Process
- Assumptions
- Executive Summary
- Australia Reprocessed Medical Devices Market Trends & Development
- Australia Reprocessed Medical Devices Market Industry Dynamics
- Drivers
- Challenges
- Australia Reprocessed Medical Devices Market Hotspot & Opportunities
- Australia Reprocessed Medical Devices Market Policies, Regulations, Product Standards
- Australia Reprocessed Medical Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenues (USD Million)
- Market Share & Analysis
- By Device Type
- Cardiovascular Devices - Market Size & Forecast 2020-2030, USD Million
- Laparoscopic Devices - Market Size & Forecast 2020-2030, USD Million
- Gastroenterology Devices - Market Size & Forecast 2020-2030, USD Million
- General Surgery Devices - Market Size & Forecast 2020-2030, USD Million
- Orthopedic Devices - Market Size & Forecast 2020-2030, USD Million
- Others - Market Size & Forecast 2020-2030, USD Million
- By Classification
- Critical - Market Size & Forecast 2020-2030, USD Million
- Non-Critical - Market Size & Forecast 2020-2030, USD Million
- Semi-Critical - Market Size & Forecast 2020-2030, USD Million
- By End User
- Hospitals - Market Size & Forecast 2020-2030, USD Million
- Diagnostic Centers - Market Size & Forecast 2020-2030, USD Million
- Ambulatory Surgical Centers - Market Size & Forecast 2020-2030, USD Million
- By Region
- North
- South
- East
- West
- By Company
- Competition Characteristics
- Company Share & Analysis
- By Device Type
- Market Size & Analysis
- Australia Cardiovascular Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Classification - Market Size & Forecast 2020-2030, USD Million
- By End User - Market Size & Forecast 2020-2030, USD Million
- Market Size & Analysis
- Australia Laparoscopic Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Classification - Market Size & Forecast 2020-2030, USD Million
- By End User - Market Size & Forecast 2020-2030, USD Million
- Market Size & Analysis
- Australia Gastroenterology Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Classification - Market Size & Forecast 2020-2030, USD Million
- By End User - Market Size & Forecast 2020-2030, USD Million
- Market Size & Analysis
- Australia General Surgery Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Classification - Market Size & Forecast 2020-2030, USD Million
- By End User - Market Size & Forecast 2020-2030, USD Million
- Market Size & Analysis
- Australia Orthopedic Devices Market Outlook, 2020-2030F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Classification - Market Size & Forecast 2020-2030, USD Million
- By End User - Market Size & Forecast 2020-2030, USD Million
- Market Size & Analysis
- Australia Reprocessed Medical Devices Market Key Strategic Imperatives for Growth & Success
- Competitive Outlook
- Company Profiles
- Stryker
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- ReNu Medical, Inc.
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Medline Industries
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Cleanpart Gmb
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Cardinal Health ANZ
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Hygia Healthcare
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- SureTek Medical
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- NEScientific, Inc.
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Medtronic PLC
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Johnson & Johnson
- Business Description
- Type Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- Stryker
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
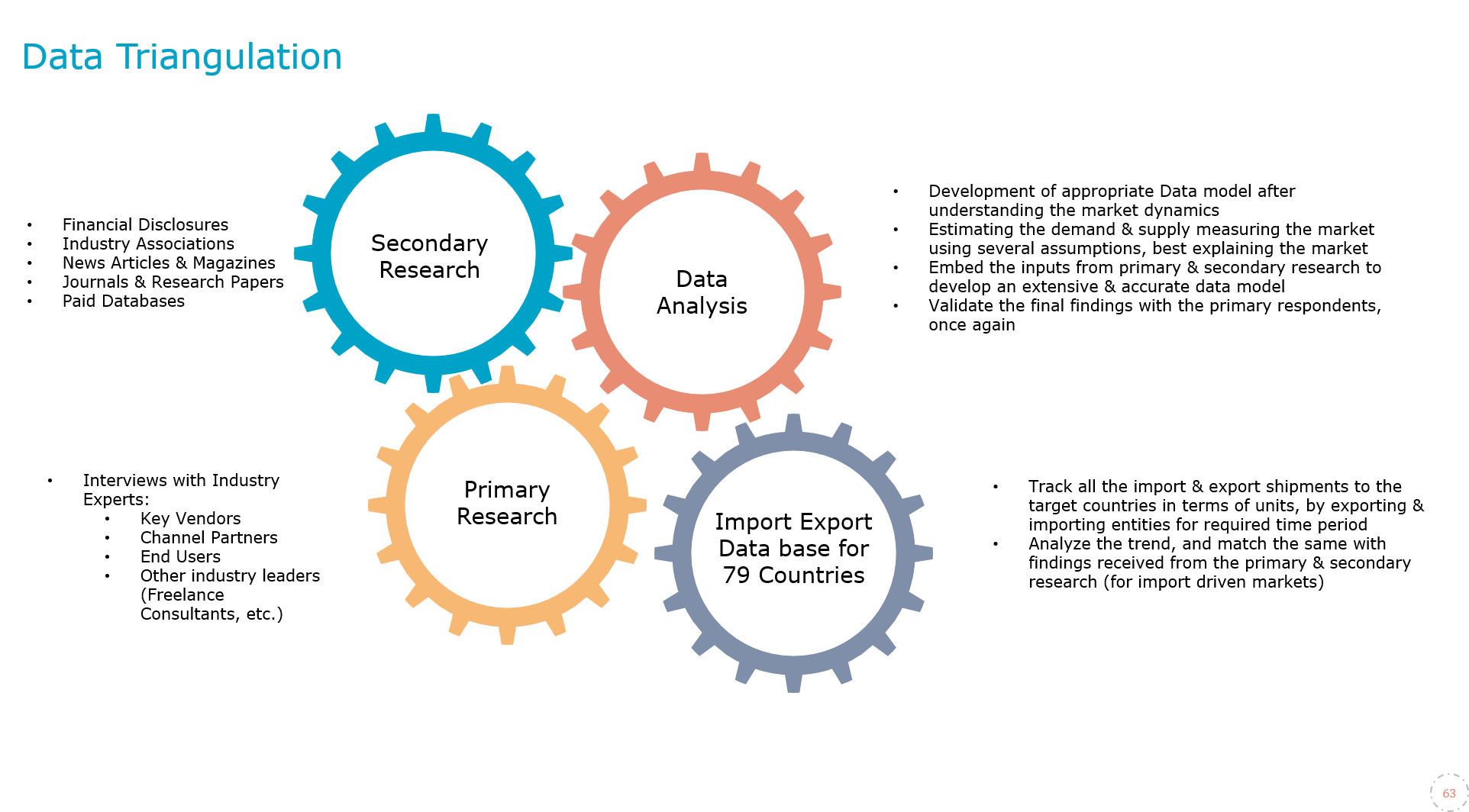
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









