
United States Active Pharmaceutical Ingredients (API) Market Research Report: Forecast (2026-2032)
United States Active Pharmaceutical Ingredients (API) Market - By Product Type (Synthetic APIs, Biotech APIs, High-Potency APIs (HPAPI), Others (Peptides, Oligonucleotides, etc.), ...By Distribution Channel (Direct Sales to Pharmaceutical Companies, Distributors and Wholesalers, Online Platforms), By Category (Generic APIs, Innovative APIs, By Type of Manufacturer (Captive Manufacturers, Merchant Manufacturers, By Application (Oncology, Cardiovascular, Diabetes, Respiratory Diseases, Others (Neurological Disorders, etc.), By End User (Pharmaceutical Manufacturers, Contract Development and Manufacturing Organizations (CDMOs), Research Institutions), and others Read more
- Healthcare
- Nov 2025
- Pages 135
- Report Format: PDF, Excel, PPT
United States Active Pharmaceutical Ingredients (API) Market
Projected 4.23% CAGR from 2026 to 2032
Study Period
2026-2032
Market Size (2025)
USD 79.98 Billion
Market Size (2032)
USD 106.87 Billion
Base Year
2025
Projected CAGR
4.23%
Leading Segments
By Product Type: Synthetic APIs
United States Active Pharmaceutical Ingredients (API) Market Size: Forecast (2026-2032)
The United States Active Pharmaceutical Ingredients (API) Market size is valued at around USD 79.98 Billion in 2025 and is projected to reach USD 106.87 Billion by 2032. Along with this, the market is estimated to grow at a CAGR of around 4.23% during the forecast period, i.e., 2026-32.
United States Active Pharmaceutical Ingredients (API) Market Outlook:
The United States Active Pharmaceutical Ingredients (API) Market is witnessing a phase of strong transformation and innovation, driven by advances in healthcare technology and the nation’s growing emphasis on high-quality pharmaceutical manufacturing. For instance, the U.S. Food and Drug Administration (FDA) has been actively promoting the adoption of advanced manufacturing methods, including continuous and modular production, to ensure consistent quality, efficiency, and faster market delivery of APIs. These initiatives are supported through the Advanced Manufacturing Technologies Designation Program, launched to accelerate next-generation pharmaceutical production.
Additionally, increasing demand for complex therapies and biologics is fostering significant progress in high-potency API (HPAPI) production across the country. For instance, the National Institutes of Health (NIH) highlights that rising cases of cancer and chronic diseases such as diabetes and cardiovascular disorders are fueling the development of targeted and personalized medications, which require specialized APIs. Similarly, major pharmaceutical firms in the U.S. are expanding their API facilities and investing in sustainable manufacturing practices to enhance capacity and reduce environmental impact.
Government-backed efforts to modernize pharmaceutical infrastructure, combined with robust R&D and rising healthcare spending, are strengthening the industry’s long-term foundation. The U.S. remains a global leader in drug innovation, and its API segment reflects this momentum, advancing through technology, quality assurance, and regulatory support. Altogether, these developments position the United States API Industry for sustained growth, greater efficiency, and continued global competitiveness during the forecast period.
United States Active Pharmaceutical Ingredients (API) Market Recent Developments:
- September 2025: AbbVie has started construction on a new active-pharmaceutical-ingredient (API) manufacturing facility in North Chicago, Illinois, to support its immunology, oncology, and neuroscience drugs. The site is expected to be fully operational by 2027 and reflects AbbVie's effort to shift more API production to the U.S. from Europe and Asia.
United States Active Pharmaceutical Ingredients (API) Market Scope:
| Category | Segments |
|---|---|
| By Product Type | Synthetic APIs, Biotech APIs, High-Potency APIs (HPAPI), Others (Peptides, Oligonucleotides, etc.), |
| By Distribution Channel | Direct Sales to Pharmaceutical Companies, Distributors and Wholesalers, Online Platforms), |
| By Category | Generic APIs, Innovative APIs, |
| By Type of Manufacturer | Captive Manufacturers, Merchant Manufacturers, |
| By Application | Oncology, Cardiovascular, Diabetes, Respiratory Diseases, Others (Neurological Disorders, etc.), |
| By End User | Pharmaceutical Manufacturers, Contract Development and Manufacturing Organizations (CDMOs), Research Institutions), and others |
United States Active Pharmaceutical Ingredients (API) Market Drivers:
Increasing Burden of Chronic Disease Driving Market Demand
The rising prevalence and severity of chronic diseases are key drivers strengthening the U.S. Active Pharmaceutical Ingredients (API) Market. According to the Centers for Disease Control and Prevention (CDC), nearly 60% of U.S. adults live with at least one chronic disease, while 40% manage two or more. Conditions such as heart disease, cancer, diabetes, and respiratory disorders remain among the leading causes of death and disability nationwide. For instance, heart disease alone accounts for about 680,980 deaths annually, and over 38 million Americans live with diabetes.
Similarly, the National Cancer Institute (NCI) estimated around 2 million new cancer cases in 2025, reflecting the increasing therapeutic demand for targeted and biologic drugs. This surge in long-term treatments has intensified the need for diverse and high-quality APIs to support continuous drug production. Consequently, pharmaceutical manufacturers are expanding their capabilities to meet this growing medical necessity, propelling sustained API market growth across the United States.
Rising Healthcare and R&D Investments
Increasing healthcare and research investments are playing a vital role in driving the growth of the U.S. Active Pharmaceutical Ingredients (API) Market. For instance, the National Institutes of Health (NIH) allocated more than USD48 billion in 2024 to biomedical and pharmaceutical research, supporting the discovery of advanced drug compounds and next-generation APIs.
Similarly, the U.S. Department of Health and Human Services (HHS), through the Biomedical Advanced Research and Development Authority (BARDA), continues to fund projects that strengthen domestic pharmaceutical development and supply capabilities. These rising public and institutional investments are promoting advanced manufacturing, accelerating clinical breakthroughs, and fostering a stronger research ecosystem, collectively propelling the long-term expansion of the U.S. API market through innovation and sustained production efficiency.
United States Active Pharmaceutical Ingredients (API) Market Trends:
Green & Sustainable API Production Gaining Momentum
Sustainability has become a central trend in the U.S. Active Pharmaceutical Ingredients (API) Industry as manufacturers focus on reducing environmental impact and improving production efficiency. The U.S. Environmental Protection Agency (EPA) promotes the adoption of green chemistry principles, encouraging the use of renewable raw materials and safer solvents to minimize chemical waste and emissions. For instance, the EPA’s Green Chemistry Challenge Awards have recognized several pharmaceutical innovations that cut hazardous solvent use by up to 830 million pounds of hazardous chemicals and solvents, while maintaining high product yield.
Additionally, leading U.S. drugmakers are increasingly employing biocatalytic and enzymatic synthesis, which reduces reaction steps and energy consumption. These eco-efficient techniques support both environmental sustainability and regulatory compliance under programs such as the Pollution Prevention Act. As a result, green and sustainable API manufacturing is emerging as a competitive advantage, aligning with national goals to promote cleaner, safer, and more resilient pharmaceutical production.
Growth of Biologic and High-Potency APIs (HPAPIs)
The rapid expansion of biologic and high-potency APIs (HPAPIs) is reshaping the U.S. pharmaceutical manufacturing landscape. For instance, biologics have accounted for over 35-40% of new drug approvals in recent years, underscoring the nation’s shift toward precision and targeted therapies. Additionally, FDA approvals for monoclonal antibodies and immunotherapies used in oncology and autoimmune disorders have significantly surged since 2020.
This trend is driving investments in high-containment and advanced manufacturing facilities capable of producing APIs with superior purity and potency. Additionally, the National Institutes of Health (NIH) continues to support R&D in biologics and complex molecules to treat rare diseases and cancers. Together, these developments highlight a decisive move toward high-value, specialized APIs, strengthening the U.S. position as a global leader in biologic and HPAPI innovation and production.
United States Active Pharmaceutical Ingredients (API) Market Challenges:
High Production and Compliance Costs Hindering Market Expansion
High production and compliance costs remain one of the biggest challenges in the United States Active Pharmaceutical Ingredients (API) Market. API manufacturers must adhere to stringent U.S. Food and Drug Administration (FDA) regulations under Current Good Manufacturing Practices (cGMP), which significantly increase operational expenses. Additionally, the Environmental Protection Agency (EPA) enforces strict emission and waste treatment standards, further adding to manufacturing expenditures.
Energy consumption for large-scale synthesis and purification processes also remains high, with average industrial utility costs in the U.S. exceeding USD9.06 per kWh, as per the U.S. Energy Information Administration, 2025. These combined regulatory, environmental, and operational expenses make domestic API production less cost-competitive than imports, particularly for small and mid-sized manufacturers.
United States Active Pharmaceutical Ingredients (API) Market (2026-32) Segmentation Analysis:
The United States Active Pharmaceutical Ingredients (API) Market Report and Forecast 2026-2032 offers a detailed analysis of the market based on the following segments:
Based on Product Type
- Synthetic APIs
- Biotech APIs
- High-Potency APIs (HPAPI)
- Others (Peptides, Oligonucleotides, etc.)
Synthetic APIs continue to dominate the United States' Active Pharmaceutical Ingredients (API) Market due to their cost-effectiveness, scalability, and well-established production infrastructure. Most pharmaceutical drugs approved by the U.S. Food and Drug Administration (FDA) are still chemically synthesized, reflecting the strong reliance on small-molecule medicines. For instance, the U.S. Chemical Safety Board notes that advances in process chemistry and automation have improved efficiency in large-scale synthesis, reducing production costs and waste. Furthermore, the ability of synthetic APIs to achieve high purity and consistent yields makes them preferred for chronic disease therapies such as cardiovascular and anti-infective drugs. This combination of economic viability, regulatory familiarity, and manufacturing maturity firmly positions synthetic APIs as the leading segment in the U.S. API market.
Based on Type of Manufacturer
- Captive Manufacturers
- Merchant Manufacturers
Captive manufacturers dominate the United States Active Pharmaceutical Ingredients (API) Market because major pharmaceutical companies continue to prioritize in-house production for quality control, security, and efficiency. For instance, Pfizer’s Kalamazoo plant produces nearly 1,200 metric tons of APIs each year, making it one of the largest single-site API facilities in the country, while AbbVie has started building an advanced API plant in North Chicago to strengthen domestic production. These facilities ensure compliance with strict FDA quality standards and reduce dependence on imports. Additionally, the U.S. Department of Health and Human Services (HHS) has encouraged companies to maintain local API production to secure national medicine supply chains. This growing focus on reliability, scale, and innovation keeps captive manufacturers at the forefront of the U.S. API market.
Leading Players of the United States Active Pharmaceutical Ingredients (API) Market:
- Pfizer Inc.
Pfizer Inc. is among the foremost pharmaceutical manufacturers in the United States, producing a broad range of APIs for therapeutic areas such as oncology, cardiovascular health, and infectious diseases. The company’s integrated R&D and large-scale manufacturing facilities enable high-purity production through advanced chemical synthesis and biotechnology.
- Merck & Co., Inc.
Merck & Co., Inc. is a key contributor to the U.S. API landscape, focusing on small-molecule and biologics-based active ingredients. The company leverages cutting-edge fermentation and purification technologies to ensure high-quality output for vaccines and specialty drugs. Additionally, Merck’s partnerships with U.S. research institutes strengthen its innovation pipeline and maintain consistent compliance with FDA and cGMP standards.
- AbbVie Inc.
AbbVie Inc. specializes in the development of complex APIs, particularly for immunology and oncology therapies. Its advanced production facilities in the United States employ continuous manufacturing systems that improve scalability and precision. Likewise, AbbVie’s ongoing R&D investments and collaborations with biotechnology firms enhance its capability to produce next-generation biologically derived APIs, reinforcing its leadership in specialty pharmaceuticals.
Amgen Inc., Thermo Fisher Scientific Inc., Catalent, Inc., Lonza Group AG, Cambrex Corporation, Johnson & Johnson, Gilead Sciences, and Others are the key players in the United States Active Pharmaceutical Ingredients (API) Market.
United States Active Pharmaceutical Ingredients (API) Market (2026-32): Regional Projection
The Northeast region of the United States maintains a dominant position in the Active Pharmaceutical Ingredients (API) Market, driven by its deep-rooted pharmaceutical heritage, advanced infrastructure, and concentration of global industry leaders. States such as New Jersey, Massachusetts, Pennsylvania, and New York collectively form the backbone of the nation’s biopharmaceutical manufacturing base. For instance, New Jersey hosts over 5,600 life sciences establishments, including eight of the world’s top ten biopharma companies, reflecting its pivotal role in drug substance and API production.
Additionally, according to the New Jersey Economic Development Authority (NJEDA), the state leads the nation in FDA-registered pharmaceutical manufacturing facilities, underscoring its regulatory and operational strength. Likewise, the Massachusetts Life Sciences Center reports continuous expansion of API-related R&D clusters around Boston and Cambridge, supported by institutions like MIT and Harvard. This combination of innovation, infrastructure, and proximity to top-tier talent reinforces the Northeast’s sustained leadership in the U.S. API Industry.
Gain a Competitive Edge with Our United States Active Pharmaceutical Ingredients (API) Market Report
- United States Active Pharmaceutical Ingredients (API) Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size & share, growth rate, competitive landscape, and key players. This comprehensive analysis helps businesses gain a holistic understanding of the market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing businesses to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- United States Active Pharmaceutical Ingredients (API) Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, businesses can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Introduction
- Product Definition
- Research Process
- Assumptions
- Executive Summary
- United States Active Pharmaceutical Ingredients (API) Market Regulations, Policies & Standards
- United States Active Pharmaceutical Ingredients (API) Market Trends & Developments
- United States Active Pharmaceutical Ingredients (API) Market Pricing Analysis
- United States Active Pharmaceutical Ingredients (API) Market Strategic Insights
- United States Active Pharmaceutical Ingredients (API) Market Dynamics
- Growth Drivers
- Challenges
- Trends
- Opportunities
- United States Active Pharmaceutical Ingredients (API) Market Hotspots & Opportunities
- United States Active Pharmaceutical Ingredients (API) Market Value Chain Analysis
- United States Active Pharmaceutical Ingredients (API) Market Outlook, 2020- 2032F
- Market Size & Analysis
- Market Revenues (USD Million)
- Market Share & Analysis
- By Product Type
- Synthetic APIs- Market Size & Forecast 2020-2032, USD Million
- Biotech APIs- Market Size & Forecast 2020-2032, USD Million
- High-Potency APIs (HPAPI)- Market Size & Forecast 2020-2032, USD Million
- Others (Peptides, Oligonucleotides, etc.)- Market Size & Forecast 2020-2032, USD Million
- By Distribution Channel
- Direct Sales to Pharmaceutical Companies- Market Size & Forecast 2020-2032, USD Million
- Distributors and Wholesalers- Market Size & Forecast 2020-2032, USD Million
- Online Platforms- Market Size & Forecast 2020-2032, USD Million
- By Category
- Generic APIs- Market Size & Forecast 2020-2032, USD Million
- Innovative APIs- Market Size & Forecast 2020-2032, USD Million
- By Type of Manufacturer
- Captive Manufacturers- Market Size & Forecast 2020-2032, USD Million
- Merchant Manufacturers- Market Size & Forecast 2020-2032, USD Million
- By Application
- Oncology- Market Size & Forecast 2020-2032, USD Million
- Cardiovascular- Market Size & Forecast 2020-2032, USD Million
- Diabetes- Market Size & Forecast 2020-2032, USD Million
- Respiratory Diseases- Market Size & Forecast 2020-2032, USD Million
- Others (Neurological Disorders, etc.)- Market Size & Forecast 2020-2032, USD Million
- By End User
- Pharmaceutical Manufacturers- Market Size & Forecast 2020-2032, USD Million
- Contract Development and Manufacturing Organizations (CDMOs)- Market Size & Forecast 2020-2032, USD Million
- Research Institutions- Market Size & Forecast 2020-2032, USD Million
- By Region
- Northeast
- Southeast
- West
- Central
- By Competitors
- Competition Characteristics
- Market Share & Analysis
- By Product Type
- Market Size & Analysis
- United States Synthetic APIs Market Outlook, 2020- 2032F
- Market Size & Analysis
- Market Revenues (USD Million)
- Market Share & Analysis
- By Distribution Channel- Market Size & Forecast 2020-2032, USD Million
- By Category- Market Size & Forecast 2020-2032, USD Million
- By End User- Market Size & Forecast 2020-2032, USD Million
- By Application- Market Size & Forecast 2020-2032, USD Million
- Market Size & Analysis
- United States Biotech APIs Market Outlook, 2020- 2032F
- Market Size & Analysis
- Market Revenues (USD Million)
- Market Share & Analysis
- By Distribution Channel- Market Size & Forecast 2020-2032, USD Million
- By Category- Market Size & Forecast 2020-2032, USD Million
- By End User- Market Size & Forecast 2020-2032, USD Million
- By Application- Market Size & Forecast 2020-2032, USD Million
- Market Size & Analysis
- United States High-Potency APIs Market Outlook, 2020- 2032F
- Market Size & Analysis
- Market Revenues (USD Million)
- Market Share & Analysis
- By Distribution Channel- Market Size & Forecast 2020-2032, USD Million
- By Category- Market Size & Forecast 2020-2032, USD Million
- By End User- Market Size & Forecast 2020-2032, USD Million
- By Application- Market Size & Forecast 2020-2032, USD Million
- Market Size & Analysis
- United States Active Pharmaceutical Ingredients (API) Market Key Strategic Imperatives for Growth & Success
- Competitive Outlook
- Company Profiles
- Pfizer Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Merck & Co., Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- AbbVie Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Amgen Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Thermo Fisher Scientific Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Catalent, Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Lonza Group AG
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Cambrex Corporation
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Johnson & Johnson
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Gilead Sciences
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- Pfizer Inc.
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
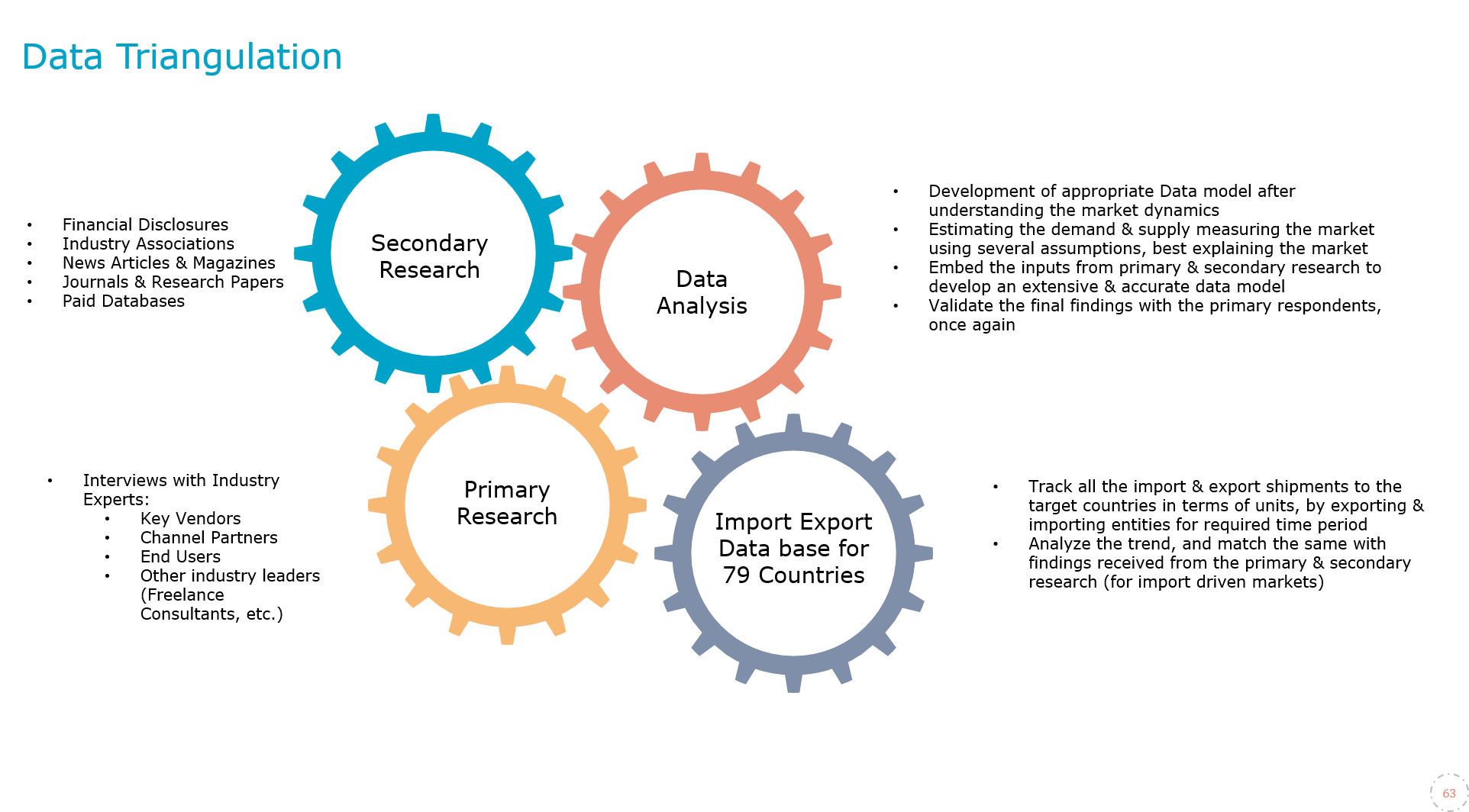
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









