
Saudi Arabia Pharmaceutical CRO Market Size: Forecast (2026-2032)
Saudi Arabia Pharmaceutical CRO Market Report Growth, Trends & Outlook - By Service Type, (Clinical Research Services, Preclinical Research Services, Data Management & Biostatistic...s, Regulatory Services, Pharmacovigilance & Safety, Medical Writing & Scientific Communication, Laboratory/Bioanalytical Testing, Site Management & Monitoring, Consulting & Quality Management, Others), By Therapeutic Area, (Oncology, Cardiovascular, Infectious Disease, Neurology, Immunology, Metabolic Disorders, Gastroenterology, Respiratory, Rare & Genetic Diseases, Others) By End User, (Biotech Firms, Medical Device Manufacturers, Academic & Research Institutes, Government & Public Healthcare Agencies), By Contract Type, (Full-Service CROs, Niche/Functional CROs, Hybrid Models), By Phase of Development, (Discovery & Preclinical, Early Phase (I-II), Late Phase (III-IV), Post-Approval & Market Surveillance) Read more
- Healthcare
- Oct 2025
- Pages 162
- Report Format: PDF, Excel, PPT
Saudi Arabia Pharmaceutical CRO Market
Projected 14.97% CAGR from 2026 to 2032
Study Period
2026-2032
Market Size (2025)
USD 223 million
Market Size (2032)
USD 448 million
Base Year
2025
Projected CAGR
14.97%
Leading Segments
By Service Type: Clinical Research Services
The Saudi Arabia Pharmaceutical CRO Market size is valued at around USD 223 million in 2025 and is projected to reach USD 448 million by 2032. Along with this, the market is estimated to grow at a CAGR of around 14.97% during the forecast period, i.e., 2026-32.
Saudi Arabia Pharmaceutical CRO Market Key Takeaways:
- According to SFDA procedural regulations, sponsors and CROs are required to register studies in the Saudi Clinical Study Registry and inform the SFDA within certain timeframes (for many study types, this is 20 working days following local IRB clearance). This tightens procedural timelines while lowering "start-up uncertainty."
- A recurrent requirement in SFDA clinical trial rules is that applicants (and frequently CROs working for foreign sponsors) have an official presence or a legal agent inside the Kingdom.
Saudi Arabia Pharmaceutical CRO Market Key Trends & Developments:
- October 09, 2025
King Faisal Specialist Hospital & Research Center (KFSHRC) launched more than 80 new studies in the areas of cancer, cardiology, and metabolic illnesses in the first half of 2025, accounting for over 48% of Saudi Arabia's clinical trials. By administering a locally produced CAR-T cell treatment to its first patient, it also accomplished a significant milestone.
- June 18, 2025
Saudi Arabia is building its first fully integrated modular Advanced Therapy Medicinal Products (ATMP) manufacturing complex in Riyadh through King Faisal Specialist Hospital & Research Center (KFSHRC) and Germfree Laboratories. The center will boost clinical research capabilities, draw international biotech partnerships, and speed up local cell and gene therapy production.
Saudi Arabia Pharmaceutical CRO Market Scope:
| Category | Segments |
|---|---|
| By Service Type | Clinical Research Services, Preclinical Research Services, Data Management & Biostatistics, Regulatory Services, Pharmacovigilance & Safety, Medical Writing & Scientific Communication, Laboratory/Bioanalytical Testing, Site Management & Monitoring, Consulting & Quality Management, Others |
| By Therapeutic Area | Oncology, Cardiovascular, Infectious Disease, Neurology, Immunology, Metabolic Disorders, Gastroenterology, Respiratory, Rare & Genetic Diseases, Others |
| By End User | Biotech Firms, Medical Device Manufacturers, Academic & Research Institutes, Government & Public Healthcare Agencies |
| By Contract Type | Full-Service CROs, Niche/Functional CROs, Hybrid Models |
| By Phase of Development | Discovery & Preclinical, Early Phase (I-II), Late Phase (III-IV), Post-Approval & Market Surveillance |

Saudi Arabia Pharmaceutical CRO Market Drivers:
Government-Led Biotech Localization and R&D Incentives
One of the main factors propelling the Saudi Arabian Pharmaceutical CRO Market Growth is the Government-led biotech localization and new R&D incentives. To lessen reliance on imports, Saudi Arabia's National Biotechnology Strategy, which was introduced in 2024, specifically encourages the localization of biologics, vaccines, and bio-manufacturing. The government is using incentives (tax cuts, facilitation, import permission acceleration) to entice biopharma R&D since it considers drug security to be a strategic priority. As an actual example, the Saudi single-use bioprocessing systems market is expected to increase significantly by 2032, reflecting the country's drive for domestic biologics and rising single-use bioprocessing investment. The need for CROs with the ability to produce assays, support bioprocesses, and handle biologics will therefore rise.
Healthcare Transformation & Institutional Expansion
The Saudi Arabia CRO Industry is growing significantly due to the healthcare transformation and institutional expansion. To increase access and innovation, Saudi Arabia is growing its digital health, healthcare infrastructure, and research capabilities under the Vision 2030 Health Sector Transformation Program. Clinical trial numbers are expected to rise as a result of governments' expanding expenditure and transforming hospitals into research facilities. For instance, Saudi Arabia's national pavilion participation at international biotech conferences (BIO 2025) demonstrates institutional drive for alliances and exposure in the life sciences. Demand for CRO services is directly fueled by this institutional impetus in all stages and regions.
Saudi Arabia Pharmaceutical CRO Market Trends:
Adoption of Modular & Flexible Biopharmaceutical Manufacturing
The adoption of modular and flexible biopharmaceutical manufacturing is changing the dynamics of the Saudi Arabia CRO Industry. The demand for agility, cost effectiveness, and quick scaling in the manufacture of biopharmaceuticals is driving a noticeable increase in the use of modular and flexible manufacturing in Saudi Arabia's contract CRO sector. A developing research ecosystem depends on modular and single-use systems because they allow for quicker facility setup, lower contamination concerns, and flexible capacity for different project sizes. Such inventions are encouraged by the National Biotechnology Strategy and the Saudi government's localization aspirations under Vision 2030 in order to support the country's clinical research and bio manufacturing infrastructure. One real-world example is the partnership between King Faisal Specialist Hospital & Research Center and Germfree Laboratories, which supported advanced therapy trials and international research collaborations by establishing the country's first modular Advanced Therapy Medicinal Products (ATMP) manufacturing campus in Riyadh.
Heightened SFDA Regulatory Oversight and GCP Inspections
The Saudi Food & Drug Authority (SFDA) is stepping up its regulatory monitoring and Good Clinical Practice (GCP) inspections of the country's Contract Research Organization (CRO) industry. The purpose of this heightened examination is to guarantee patient safety, trial integrity, and conformity to global norms. Sponsors and CROs are subject to certain regulations, which include data management, informed consent processes, paperwork, and site monitoring. The Kingdom's desire to increase clinical trial credibility and draw in international pharmaceutical partnerships is what is driving the increased regulatory attention. The SFDA's extended inspection program, which emphasizes compliance and data dependability and is implemented across many institutions performing Phase I–III studies, is a real-world example.
Saudi Arabia Pharmaceutical CRO Market Challenges:
Complex & Evolving Regulatory Landscape
Complex and frequent changes in regulatory regulations provide difficulties for the Saudi CRO industry. Trial beginning may be delayed by the procedural complexities created by several levels of monitoring, such as import permissions, institutional review boards, SFDA clearances, and local representation requirements. Processes, SOPs, and compliance frameworks must be continually adjusted by CROs since rules are often changed to conform to worldwide standards. The prolonged SFDA inspection program throughout Phase I–III trials provides a practical example, revealing discrepancies in site preparation and paperwork. For both local and foreign CROs, this changing regulatory environment can impede market development and raise operating expenses.
Saudi Arabia Pharmaceutical CRO Market (2026-32) Segmentation Analysis:
The Saudi Arabia Pharmaceutical CRO Market Report and Forecast 2026-2032 offers a detailed analysis of the market based on the following segments:
Based on Service Type
- Clinical Research Services
- Preclinical Research Services
- Regulatory Services
- Data Management & Biostatistics
- Pharmacovigilance & Safety
- Medical Writing & Scientific Communication
- Laboratory/Bioanalytical Testing
- Site Management & Monitoring
- Consulting & Quality Management
- Others
The Clinical Research Services segment holds the top spot in the Saudi Arabia Pharmaceutical CRO Market. Clinical trial services, including early-stage and post-marketing research, are the main driver of the Saudi Arabia CRO industry. The crucial role clinical trials play in the drug development lifecycle, which makes it easier to assess the safety and effectiveness of novel treatments, is the reason for this dominance. This market has been further driven by the rising need for enhanced clinical trial services as well as the increased focus on patient safety and regulatory compliance.
Based on Therapeutic Area
- Oncology
- Cardiology
- Infectious Diseases
- Metabolic Disorders (Diabetes, etc.)
- Neurology
- Immunology
- Gastroenterology
- Respiratory
- Rare & Genetic Diseases
- Others
Oncology dominates the Saudi Arabia CRO Industry. This is due to a sudden increase in cancer cases and large government investments in cancer research and treatment facilities. The need for specialist research and clinical trials in this area has increased due to the rising incidence of cancer, which is driven by factors including aging populations and changes in lifestyle. The nation's determination to defeat cancer is demonstrated, for example, by the creation of specialist oncology facilities and the significant financing provided for cancer research projects. cancer is now the most popular therapeutic area in the market due to the emphasis on cancer research, which has increased demand for CRO services.
Leading Manufacturers of the Saudi Arabia Pharmaceutical CRO Market:
- ArabMed CRO
ArabMed CRO, a well-known regional full-service contract research organization with its headquarters located in Riyadh, Saudi Arabia, was founded in 2005. In response to the increasing need for clinical research services in the GCC, ArabMed CRO, which specializes in regulatory affairs, clinical monitoring, and pharmacovigilance, has extended its activities throughout the GCC, including Egypt and Jordan. They are a major participant in the Saudi CRO industry because of their broad experience and regional reach.
- Balsam Clinical Research
Balsam Clinical Research is a Saudi contract research organization headquartered in Riyadh that was established in 2021. The business provides a wide range of clinical research services, such as regulatory support, clinical trial administration, biostatistics, and medical writing. Balsam Clinical Research is dedicated to upholding strict international guidelines to provide the Kingdom with dependable and successful clinical studies.
- Mesned Clinical Research Organization
Mesned CRO is a dynamic full-service contract research organization with its headquarters located in Riyadh, Saudi Arabia. Mesned CRO was founded to offer strategic and high-quality services to academic research institutions and the healthcare industry in the GCC region. Its areas of expertise are clinical trial administration and product registration.
Clinart MENA, King Faisal Specialist Hospital & Research Center (KFSHRC) Clinical Trials Unit, Quintiles IMS (IQVIA), ICON plc, SGS Life Sciences, WuXi AppTec, Thermo Fisher Scientific, LabCorp Drug Development, Syneos Health, Medpace, Covance, PPD (Part of Thermo Fisher), and others are the key players of the Saudi Arabia Pharmaceutical CRO Market.
Saudi Arabia Pharmaceutical CRO Market (2026-32): Regional Projection
The Saudi Arabia Pharmaceutical CRO Market is dominated by the Riyadh Region since it is the country's capital and a center for government agencies, medical facilities, and research facilities. Clinical trials and research operations are drawn to Riyadh because of its infrastructure and concentration of medical facilities. The King Faisal Specialist Hospital & Research Center in Riyadh, for example, is actively engaged in innovative clinical studies and has partnerships with foreign organizations, like as Texas A&M University for cell therapy research. The area is positioned as the top market participant in Saudi Arabia, owing to the concentration of resources and knowledge in Riyadh, which creates a situation that is favorable for the expansion of contract CRO services.
Gain a Competitive Edge with Our Saudi Arabia Pharmaceutical CRO Market Report
- Saudi Arabia Pharmaceutical CRO Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size & share, growth rate, competitive landscape, and key players. This comprehensive analysis helps businesses gain a holistic understanding of the market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing businesses to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- Saudi Arabia Pharmaceutical CRO Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, businesses can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Introduction
2.1. Product Definition
2.2. Research Process
2.3. Assumptions - Executive Summary
- Saudi Arabia Pharmaceutical CRO Market Policies, Regulations, and Product Standards
- Saudi Arabia Pharmaceutical CRO Market Supply Chain Analysis
- Saudi Arabia Pharmaceutical CRO Market Trends & Developments
- Saudi Arabia Pharmaceutical CRO Market Dynamics
- Growth Drivers
- Challenges
- Saudi Arabia Pharmaceutical CRO Market Hotspot & Opportunities
- Saudi Arabia Pharmaceutical CRO Market Outlook, 2020-2032
- Market Size & Outlook
- By Revenues (USD Million)
- By Volume (Million Units or Projects)
- Market Share & Outlook
- By Service Type– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Clinical Research Services – Market Size & Forecast 2020-2032, USD Million & Million Projects
- Phase I– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Phase II– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Phase III– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Phase IV– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Preclinical Research Services– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Data Management & Biostatistics– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Regulatory Services – Market Size & Forecast 2020-2032, USD Million & Million Projects
- Pharmacovigilance & Safety– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Medical Writing & Scientific Communication– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Laboratory/Bioanalytical Testing– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Site Management & Monitoring– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Consulting & Quality Management– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Others
- Clinical Research Services – Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Therapeutic Area– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Oncology– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Cardiovascular– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Infectious Disease– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Neurology– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Immunology– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Metabolic Disorders– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Gastroenterology– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Respiratory– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Rare & Genetic Diseases– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Others
- By End User– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Biotech Firms– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Medical Device Manufacturers– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Academic & Research Institutes– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Government & Public Healthcare Agencies– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Contract Type– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Full-Service CROs– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Niche/Functional CROs– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Hybrid Models– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Phase of Development– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Discovery & Preclinical– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Early Phase (I-II) – Market Size & Forecast 2020-2032, USD Million & Million Projects
- Late Phase (III-IV) – Market Size & Forecast 2020-2032, USD Million & Million Projects
- Post-Approval & Market Surveillance– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Region
- Riyadh (Central Region)
- Jeddah (Western Region)
- Dammam & Khobar (Eastern Region)
- Medina (Northwestern Region)
- Others (Southern & Peripheral Areas)
- By Company
- Company Revenue Shares
- Competitor Characteristics
- By Service Type– Market Size & Forecast 2020-2032, USD Million & Million Projects
- Market Size & Outlook
- Saudi Arabia Clinical Research Services Market Outlook, 2020-2032
- Market Size & Outlook
- By Revenues (USD Million)
- By Volume (Number of Trials or Projects)
- Market Share & Outlook
- By Phase – Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Therapeutic Area– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By End-User– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Region
- Market Size & Outlook
- Saudi Arabia Preclinical & Laboratory Services Market Outlook, 2020-2032
- Market Size & Outlook
- By Revenues (USD Million)
- By Volume (Number of Studies)
- Market Share & Outlook
- By Type– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By End-User– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Region
- Market Size & Outlook
- Saudi Arabia Data Management & Regulatory Services Market Outlook, 2020-2032
- Market Size & Outlook
- By Revenues (USD Million)
- By Number of Projects/Clients
- Market Share & Outlook
- By Service Type– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By End-User– Market Size & Forecast 2020-2032, USD Million & Million Projects
- By Region
- Market Size & Outlook
- Saudi Arabia Pharmaceutical CRO Market Key Strategic Imperatives for Success & Growth
- Competition Outlook
- Company Profiles
- ArabMed CRO (Saudi Arabia)
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Balsam Clinical Research (Saudi Arabia)
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Mesned CRO (Saudi Arabia)
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Clinart MENA
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- King Faisal Specialist Hospital & Research Centre (KFSHRC) Clinical Trials Unit
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Quintiles IMS (IQVIA)
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- ICON plc
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- SGS Life Sciences
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- WuXi AppTec
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Thermo Fisher Scientific
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- LabCorp Drug Development
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Syneos Health
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Medpace
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Covance
- Business Description
- Service Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- ArabMed CRO (Saudi Arabia)
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
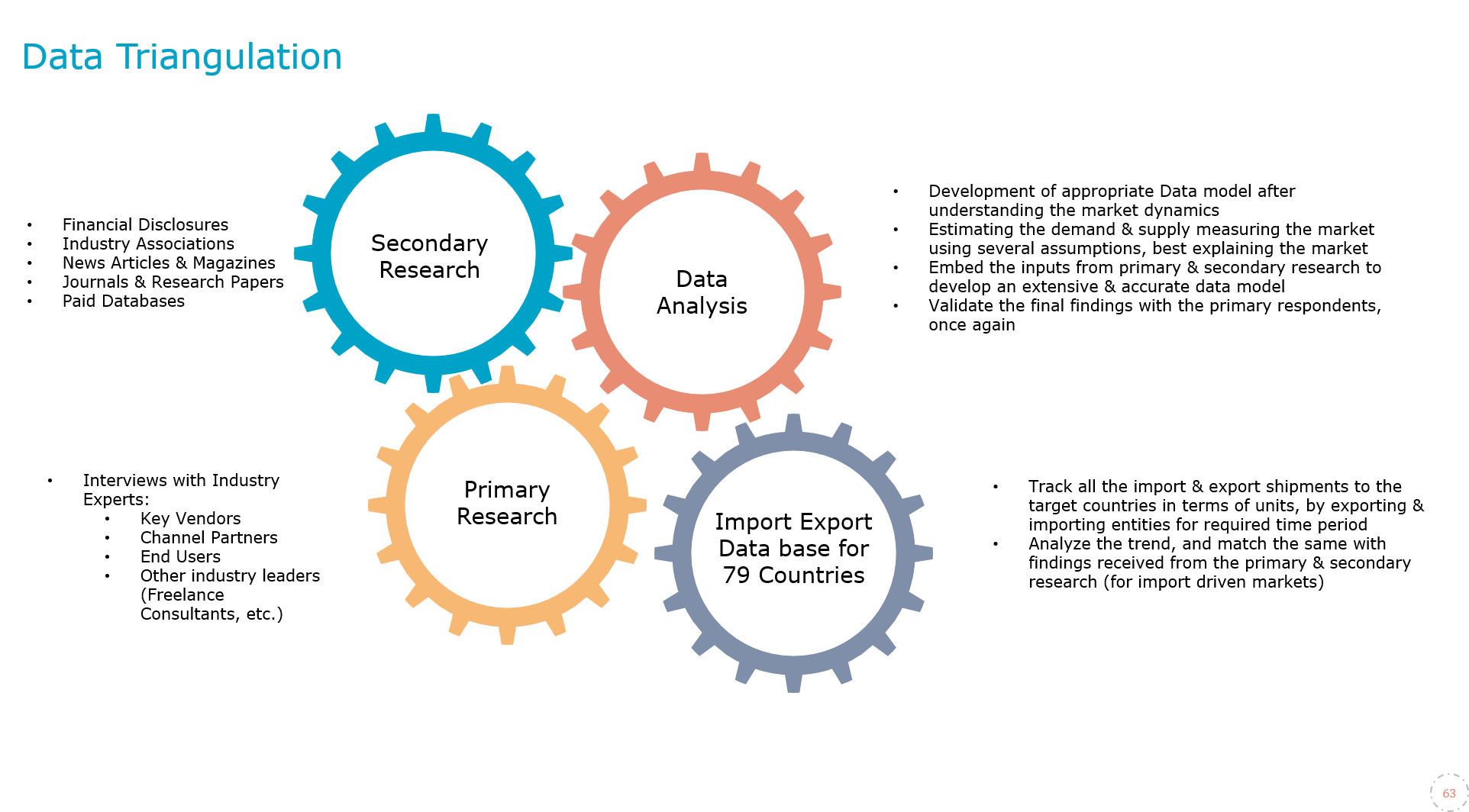
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









