
Global Transthyretin Amyloidosis Treatment Market Research Report: Forecast (2026-2032)
Transthyretin Amyloidosis Treatment Market - By Type (ATTR amyloidosis with polyneuropathy, ATTR amyloidosis with cardiomyopathy), By Disease Type (Hereditary Transthyretin Amyloid...osis, Polyneuropathy, Cardiomyopathy, Mixed type, Wild-type transthyretin amyloidosis), By Drug Type (Tafamidis, Patisiran, Inotersen, Other drug types), By Route of Administration (Oral, Parental), By Distribution Channel (Hospital pharmacy, Retail drug stores, Online pharmacy), and others Read more
- Healthcare
- Nov 2025
- Pages 163
- Report Format: PDF, Excel, PPT
Global Transthyretin Amyloidosis Treatment Market
Projected 12.70% CAGR from 2026 to 2032
Study Period
2026-2032
Market Size (2025)
USD 5.88 Billion
Market Size (2032)
USD 13.58 Billion
Largest Region
2025
Projected CAGR
12.70%
Leading Segments
By Drug Type: Tafamidis
Global Transthyretin Amyloidosis Treatment Market Size: Forecast (2026-2032)
The Global Transthyretin Amyloidosis Treatment Market size is valued at around USD5.88 billion in 2025 and is projected to reach USD13.58 billion by 2032. Along with this, the market is estimated to grow at a CAGR of around 12.70% during the forecast period, i.e., 2026-32.
Global Transthyretin Amyloidosis Treatment Market Outlook:
The Global Transthyretin (TTR) Amyloidosis Treatment Market landscape is expanding steadily as disease awareness, diagnostic capacity, and access to RNA-based and stabilizer therapies improve worldwide. For instance, ATTR-CM prevalence continues to dominate the region, like the United States, estimated approximately 120,000 people may have ATTR-CM, though most remain undiagnosed.
Moreover, recent commercial launches are reshaping treatment access. For instance, Ionis Pharmaceuticals’ eplontersen, co-commercialized with AstraZeneca, received U.S. FDA approval in 2024 for ATTR polyneuropathy, strengthening competition against Pfizer’s tafamidis, which generated USD3.3 billion in 2023 global sales.
Furthermore, growing retail distribution networks are also supporting therapy accessibility. For instance, the U.S. National Association of Chain Drug Stores (NACDS) reports over 40,000 retail drug stores nationally in 2024, improving specialty-drug dispensing penetration. Similarly, online pharmacy adoption continues to rise, with the FDA noting accelerated enrollment in regulated digital pharmacies, driven by chronic-disease prescription demand and home-delivery trends.
Overall, rising diagnostic infrastructure combined with new RNA, antisense, and stabilizer therapies is expected to keep expanding global treatment uptake and geographic reach.
Global Transthyretin Amyloidosis Treatment Market Recent Developments:
- June 2025: The European Commission approved vutrisiran (AMVUTTRA) for patients with either wild-type or hereditary ATTR cardiomyopathy. This approval means the treatment can now be prescribed across all EU countries. It expands access to an RNA-based therapy that lowers transthyretin levels and helps slow heart damage in ATTR-CM patients. The decision was based on strong clinical results from the HELIOS-B study.
- February 2024: The FDA granted Fast Track designation to eplontersen, developed by Ionis and AstraZeneca, for treating transthyretin amyloidosis with cardiomyopathy (ATTR-CM). This designation helps speed up the review and development process because ATTR-CM is a serious, progressive condition with limited treatment options. Fast Track status allows closer FDA guidance and faster access to new clinical data, supporting quicker progress toward approval.
Global Transthyretin Amyloidosis Treatment Market Scope:
| Category | Segments |
|---|---|
| By Type | ATTR amyloidosis with polyneuropathy, ATTR amyloidosis with cardiomyopathy), |
| By Disease Type | Hereditary Transthyretin Amyloidosis, Polyneuropathy, Cardiomyopathy, Mixed type, Wild-type transthyretin amyloidosis), |
| By Drug Type | Tafamidis, Patisiran, Inotersen, Other drug types), |
| By Route of Administration | Oral, Parental), |
| By Distribution Channel | Hospital pharmacy, Retail drug stores, Online pharmacy), and others |
Global Transthyretin Amyloidosis Treatment Market Drivers:
Increasing Investments for Rare-Disease Research and Clinical Infrastructure
Growing investment in rare-disease research and clinical infrastructure is accelerating progress in transthyretin amyloidosis (ATTR) treatment worldwide. The U.S. National Institutes of Health (NIH) remains the largest public funder of rare-disease research, investing about USD26 million for rare disease conditions, including amyloidosis.
Additionally, governments across Europe and Asia have also expanded rare-disease infrastructure. In September 2024, the European Commission announced that the ERDERA Partnership will mobilize a total of USD 410.4 million to support rare-disease research, covering prevention, diagnosis, and treatment efforts, supporting biomarker discovery, advanced imaging, and precision-medicine tools across EU tertiary hospitals.
Moreover, Asia is investing as well. Japan’s AMED allocated more than USD125 million in 2024 to diseases, including rare-disease genomics and protein-misfolding research, strengthening amyloidosis-related discovery programs. Meanwhile, the Canadian Institutes of Health Research (CIHR) committed USD22.75 million to rare-disease diagnostics and precision-therapy research in 2023–24.

Global Transthyretin Amyloidosis Treatment Market Trends:
Shift Toward Next-Generation RNA & Digital Diagnostic Tools
A major trend shaping the ATTR treatment landscape is the rapid shift toward next-generation RNA-based medicines. Companies such as Alnylam and Ionis are developing newer RNA therapies designed for longer durability and easier dosing. For instance, in March 2025, the FDA expanded approval for vutrisiran (Amvuttra) to treat ATTR cardiomyopathy, widening RNA-interference options for patients in the United States. This regulatory milestone reflects growing physician confidence in RNA-based therapies because of their proven ability to reduce transthyretin production and slow disease progression.
Digital diagnostic tools are reinforcing this shift by improving early detection. For example, AI-assisted ECG analysis used in leading cardiac hospitals helps identify subtle patterns linked to wild-type ATTR. Similarly, advances in nuclear imaging and AI-enhanced mapping are becoming important tools for earlier and more accurate diagnosis.
Global Transthyretin Amyloidosis Treatment Market Challenges:
High Initial Investment and Production Costs
High therapy costs remain one of the biggest barriers to ATTR treatment accessibility worldwide. Tafamidis (Vyndaqel or Vyndamax) carries a U.S. list price of approximately USD 225,000 per patient per year, according to the Institute for Clinical and Economic Review (ICER), making it one of the most expensive cardiovascular drugs ever assessed by the organization. Similarly, RNA-based therapies such as patisiran and inotersen exceed USD 450,000 per year based on publicly listed U.S. wholesale acquisition costs. These price levels are far beyond the annual healthcare spending per capita in most middle-income regions, sharply limiting patient access.
Additionally, the registration timelines further complicate affordability. For example, several emerging economies like India, Brazil, Mexico, Iran, and many more take 2 to 5 years longer than the U.S. or EU to approve high-cost orphan drugs, delaying inclusion into national insurance schemes. Diagnostic infrastructure gaps also heighten cost burdens. Combined with the need for ongoing monitoring for RNA-based treatments, these financial and structural barriers significantly limit equitable therapy access globally.
Global Transthyretin Amyloidosis Treatment Market (2026-32) Segmentation Analysis:
The Global Transthyretin Amyloidosis Treatment Market Report and Forecast 2026-2032 offers a detailed analysis of the market based on the following segments:
Based on Drug Type
- Tafamidis
- Patisiran
- Inotersen
- Others
Tafamidis dominates the global transthyretin amyloidosis (ATTR-CM) treatment industry because it is one of the most widely approved and adopted therapies worldwide, supported by strong clinical evidence and broad regulatory acceptance. It holds formal approval in major regions, including the United States, European Union, Canada, Japan, and Australia, giving it unmatched global reach. This wide regulatory footprint allowed tafamidis to become the foundational, first-line therapy in many healthcare systems long before newer RNA-based drugs entered the market.
For instance, the drug was approved in Japan in 2019, enabling clinicians across the country to use tafamidis for transthyretin-related cardiomyopathy and polyneuropathy. Japanese cardiac centers and rare-disease networks rapidly integrated the therapy into treatment pathways, strengthening its uptake across both hereditary and wild-type ATTR forms. This contributed significantly to tafamidis’ position as the most established global stabilizer therapy.
Because tafamidis is an oral, once-daily medication with proven survival benefits and requires no infusion centers or complex monitoring, it continues to be preferred by cardiologists and patients. Its widespread approvals, clinical convenience, and long-standing real-world use collectively ensure it remains the dominant drug category in the global ATTR treatment market.
Based on Distribution Channel
- Hospital pharmacy
- Retail drug stores
- Online pharmacy
Hospital pharmacies dominate this market because most advanced diagnostics and specialty medicines for this disease are tightly linked to hospital-based care. For instance, in the United States, more than 6,000 hospitals operate with pharmacy services integrated into cardiology and rare-disease programs, enabling safe handling of RNA-based drugs and stabilizers used for ATTR.
Similarly, China’s more than 38,000 hospitals provide the country’s primary access point for complex therapies, as nuclear imaging and cardiac-MRI, essential for diagnosing ATTR-CM, are predominantly available only in tertiary hospital settings. This pattern is consistent across other major healthcare markets. Japan’s thousands of hospitals support high volumes of cardiac and neurology care, with hospital pharmacies overseeing storage, temperature-controlled handling, and coordination of infusion-based therapies.
Moreover, in Germany and France, hospital pharmacies remain central to managing high-cost or specialist medicines, including the drugs treating transthyretin amyloidosis. Both countries maintain large hospital networks, around 1,874 hospitals in Germany, that routinely dispense therapies requiring strict monitoring. These large networks of hospital pharmacies are contributing to the growth of this market.
Leading Players of the Global Transthyretin Amyloidosis Treatment Market:
·Acrotech Biopharma LLC (Established 2018, USA)
Acrotech Biopharma focuses on developing and commercializing oncology and rare-disease therapeutics. Its portfolio of specialty medicines positions the company as an emerging participant in niche genetic and protein-misfolding disorders. Additionally, its emphasis on targeted biologics and patient-centric therapies supports the growing need for advanced solutions in TTR amyloidosis management.
·Alnylam Pharmaceuticals Inc. (Established 2002, USA)
Alnylam is a global leader in RNA interference (RNAi) therapeutics and one of the most influential companies in the TTR amyloidosis landscape. It developed the world’s first RNAi-based treatment for hereditary transthyretin-mediated amyloidosis, establishing a new therapeutic paradigm. Likewise, its continued investment in gene-silencing and next-generation RNA platforms reinforces its dominant market position.
·AstraZeneca plc (Established 1999, UK)
AstraZeneca is a multinational biopharmaceutical innovator with strong capabilities across cardiovascular, renal, metabolic, and rare diseases. The company’s research in protein stabilization, cardiomyopathy, and precision medicines aligns with the evolving treatment approaches for TTR amyloidosis. Additionally, its strategic collaborations in rare-disease therapeutics strengthen its influence in this clinical domain.
Astellas Pharma Inc., Bristol-Myers Squibb Company, Ionis Pharmaceuticals Inc., Johnson & Johnson, Prothena Biosciences Limited, Pfizer Inc., SOM Innovation Biotech SL, and Others are the key players in the Global Transthyretin Amyloidosis Treatment Market.
Global Transthyretin Amyloidosis Treatment (2026-32): Regional Projection
North America leads the Global Transthyretin Amyloidosis Treatment Market because it combines early regulatory approvals, concentrated clinical capacity, and expanding diagnosed patient volumes. This is due to the U.S. was among the first to authorize disease-modifying drugs. For instance, patisiran (Onpattro) received FDA approval in Aug 2018, similarly, inotersen (Tegsedi) got approved in Oct 2018, and tafamidis (Vyndaqel/Vyndamax) for ATTR-CM in May 2019 in the United States, creating an early treatment baseline.
Similarly, Canada closely followed with regulatory actions that expanded North America’s collective treatment capacity. Health Canada approved Vyndaqel (tafamidis) on January 20, 2020, for adult patients with wild-type or hereditary ATTR-CM. Similarly, Onpattro (patisiran) was approved in Canada for hereditary ATTR polyneuropathy (hATTR-PN), ensuring RNA-interference therapies became available beyond the U.S.
Additionally, recent regulatory progress further strengthens the regional dominance in this market, as Alnylam’s vutrisiran (Amvuttra) received USFDA expansion for ATTR-CM in March 2025, widening RNAi options for cardiomyopathy patients in the country. Diagnosis rates and specialist centers concentrate in North America. These factors explain North America’s dominant position in the ATTR treatment industry.
Gain a Competitive Edge with Our Global Transthyretin Amyloidosis Treatment Market Report
- Global Transthyretin Amyloidosis Treatment Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size & share, growth rate, competitive landscape, and key players. This comprehensive analysis helps businesses gain a holistic understanding of the market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing businesses to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- Global Transthyretin Amyloidosis Treatment Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, businesses can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Introduction
- Product Definition
- Research Process
- Assumptions
- Executive Summary
- Global Transthyretin Amyloidosis Treatment Market Policies, Regulations, Product Standards
- Global Transthyretin Amyloidosis Treatment Market Trends & Development
- Global Transthyretin Amyloidosis Treatment Market Dynamics
- Growth Drivers
- Challenges
- Trends
- Opportunities
- Global Transthyretin Amyloidosis Treatment Market Hotspot & Opportunities
- Global Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- ATTR amyloidosis with polyneuropathy
- ATTR amyloidosis with cardiomyopathy
- By Disease Type- (USD Million)
- Hereditary Transthyretin Amyloidosis
- Polyneuropathy
- Cardiomyopathy
- Mixed type
- Wild-type transthyretin amyloidosis
- Hereditary Transthyretin Amyloidosis
- By Drug Type- (USD Million)
- Tafamidis
- Patisiran
- Inotersen
- Other drug types
- By Route of Administration
- Oral
- Parental
- By Distribution Channel
- Hospital pharmacy
- Retail drug stores
- Online pharmacy
- By Region
- North America
- South America
- Europe
- Middle East & Africa
- Asia-Pacific
- By Company
- Competition Characteristics
- Market Share & Analysis
- By Type- (USD Million)
- Market Size & Analysis
- North America Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- By Country
- The US
- Canada
- Mexico
- The US Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Canada Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Mexico Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Market Size & Analysis
- South America Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- By Country
- Brazil
- Argentina
- Rest of South America
- Brazil Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Argentina Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Market Size & Analysis
- Europe Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- By Country
- The UK
- Italy
- Germany
- France
- Spain
- Rest of Europe
- The US Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Italy Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Germany Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- France Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Spain Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Market Size & Analysis
- Middle East & Africa Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- By Country
- Saudi Arabia
- UAE
- South Africa
- Rest of Middle East & Africa
- Saudi Arabia Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- UAE Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- South Africa Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Market Size & Analysis
- Asia-Pacific Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- By Country
- China
- Japan
- India
- South Korea
- Australia
- China Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Japan Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- India Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- South Korea Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Australia Transthyretin Amyloidosis Treatment Market Outlook, 2022-2032F
- Market Size & Analysis
- By Revenue (USD Million)
- Market Share & Analysis
- By Type- (USD Million)
- By Disease Type- (USD Million)
- By Route of Administration - (USD Million)
- By Drug Type - (USD Million)
- By Distribution Channel - (USD Million)
- Market Size & Analysis
- Market Size & Analysis
- Global Transthyretin Amyloidosis Treatment Market Key Strategic Imperatives for Success & Growth
- Competition Outlook
- Company Profiles
- Acrotech Biopharma, LLC
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Alnylam Pharmaceuticals Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- AstraZeneca Plc
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Astellas Pharma Inc
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Bridge-Myers Squibb Company
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Ionis Pharmaceuticals, Inc
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Johnson and Johnson
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Prothena Bioscience Limited
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Pfizer Inc.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- SOM Innovation Biotech, SL
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- Acrotech Biopharma, LLC
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
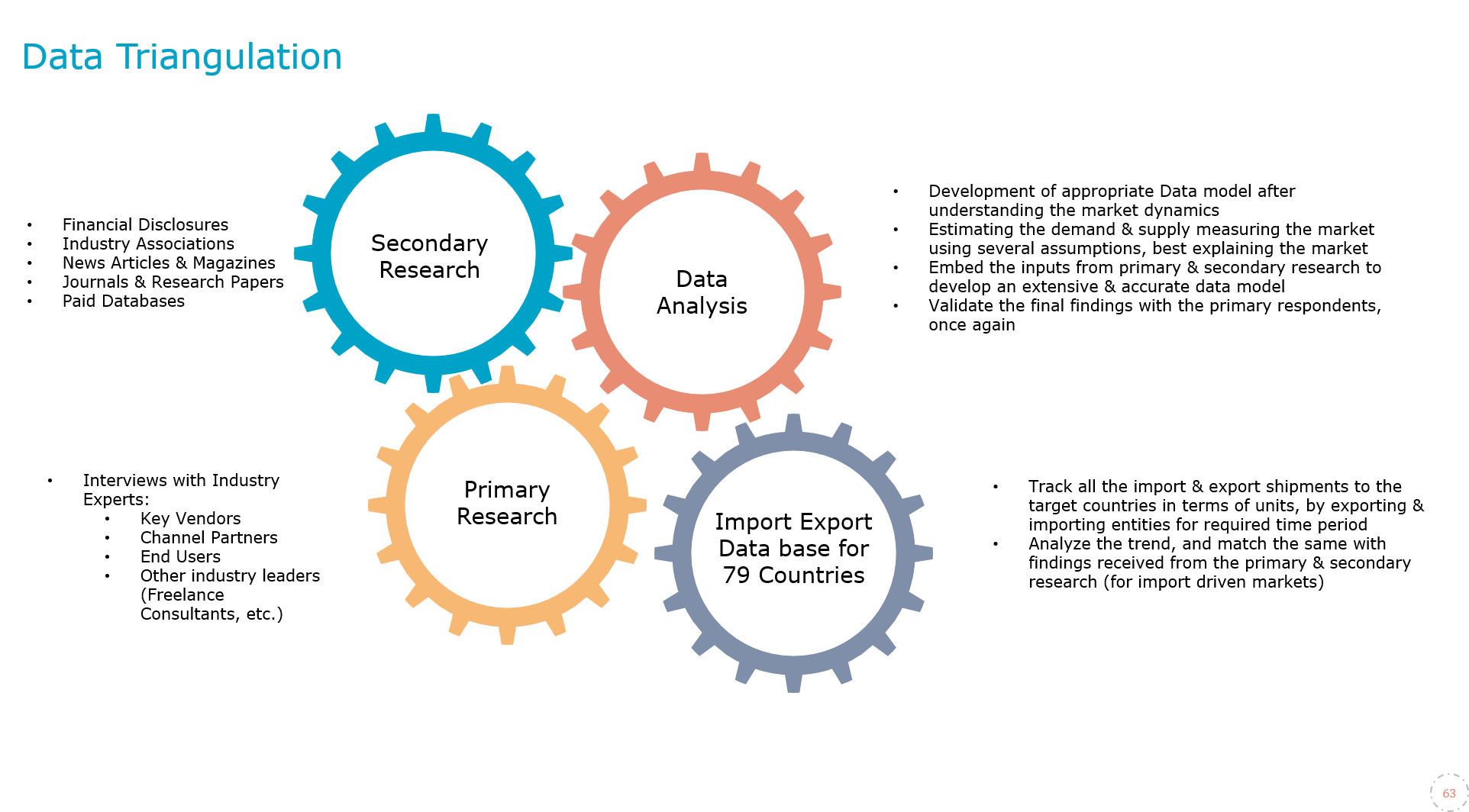
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









