
Global Single Use System in Biopharma Manufacturing Market Research Report: Forecast (2022-27)
By Bioreactors (Less than 1000L, 1000-2000L, More than 2000L), By Product (Single Use Sampling System, Automated Sampling Solutions, Off-line Single Use Sampling Solutions, Reusabl...e Manual Sampling Solutions), By Bioprocess Containers (Containers, Connectors, Tubing), Bioreactors, Mixers, Bottles (Bottles, Bottle Assemblies), Transfer Sets (Single Use Connectors, Single Use Aseptic Connectors, Disconnectors, Single Use Aseptic Disconnectors, Tubes & Assemblies, Biowelder/Biosealer), Others (Single use sensors, disposable filter cartridges), By Application (Filtration (Disposable filter Cartridges, Single use filtration bags), Storage (Media and Buffer, Freeze & Thaw)(Bioprocess Containers, Bottles, Equipment), Cell Culture (Single use Sampling system, Bioreactor, Bioprocess Containers), Mixing (Mixers), Purification (Membrane Adsorbers, Depth Filters), Aseptic Filling (Bioprocess Containers, Bottles, Transfer Sets), By Bioprocessing (Small-scale, Mid-scale, Large-scale), By Modality (Protein & Monoclonal Antibody (Mab), Cell Therapy, Gene Therapy, Conventional Vaccine, mRNA), By Component (Drug Substance, Drug Product), By Region (North America, South America, Europe, Middle East & Africa, Asia-Pacific) Read more
- Healthcare
- Apr 2022
- Pages 281
- Report Format: PDF, Excel, PPT
Market Definition
Single-use systems (SUS) refers to biopharmaceutical manufacturing (bioprocessing) equipment designed to be used once (or for a single manufacturing campaign) and then discarded. Generally, SUS equipment is composed primarily of plastic components that have been sealed and sterilized using gamma irradiation
Market Insights
The Global Single Use System in Biopharma Manufacturing market is projected to grow at a CAGR of around 20.59% during the forecast period, 2022-27. The growth can be attributed to increase in production of vaccines, rising research & development activities, growing investments in the biopharma industry, etc. The global single use system in the biopharma market experienced significant growth in historical years, i.e., 2017-21, due to the rapid evolution of the technological & research landscape in the fields of biopharma manufacturing.
| Report Coverage | Details |
|---|---|
| Study Period | Historical Data: 2017-20 |
| Base Year: 2021 | |
| Forecast Period: 2022-27 | |
| CAGR (2022-2027) | 20.59% |
| Regions Covered | North America: The US, Canada |
| Europe: Germany, The UK, France, Rest of Europe | |
| Asia-Pacific: China, India, Japan, South Korea, Rest of Asia-Pacific | |
| South America: Brazil, Rest of South America | |
| Middle East & Africa: Saudi Arabia, South Africa, Rest of The Middle East & Africa | |
| Key Companies Profiled |
Sartorius AG (Sartorius Stedim Biotech), Entegris, Pall Corporation, Eppendorf, Corning Incorporated, ThermoFisher Scientific, PBS Biotech Inc., Lonza, Merck KGaA, Danaher Corporation, Kühner AG, Rentschler Biopharma SE, Getinge AB, Others |
| Unit Denominations | USD Million/Billion |
The emergence of COVID-19 impacted the biopharmaceutical industry both positively & negatively as most of the biopharma companies & research organizations initially struggled for the development of vaccines against the SARS-CoV-2 virus. As per the Pharmaceutical Research and Manufacturers of America, during January 2021, around 1,750 clinical trials were reported to be in the progress phase & out of which 420 belong to the US. These trials were operationalized for the development of vaccines & drugs in order to provide treatment for the COVID-19 virus. This, in return, has increased the demand for single use bioprocessing systems to be used in laboratories, biopharma companies, research institutes, etc., to cater to the growing demand for vaccines around the world. Consequently, there has been a constant rise in the production & utilization of single use bioprocessing systems.

Impact of COVID-19 on Global Single Use System Market
The spread of the COVID-19 was confirmed globally by the end of March 2020, resulting in greater concern and awareness in the biotechnology industry, which led to more investment & manufacturing of biologics across the globe. Additionally, the market for single use systems witnessed increased demand in the year 2020, due to a rise in the number of clinical trials during the development of vaccines and increase in the pre-commercial product manufacturing globally.
In mid of 2020, numerous clinical biotech and healthcare research organizations, such as Pfizer, Bristol-Myers Squibb, Provention Bio, among other shifted their focus towards the development of the COVID-19 vaccine and slowing down their clinical trials of the conventional vaccine. This resulted in the rising R&D of the companies that were related to the clinical production and large-scale manufacturing of COVID-19 vaccines. Hence, the demand for single use systems during the development of vaccines observed to grow exponentially across the globe. In addition, drug discovery using single use technology has become a major application for the manufacturing of vaccines during the pandemic. The single use system helps to reduce time due to pre-sterilization and, therefore, improve accuracy during clinical trials. Thus, it helped to produce & develop vaccine for COVID-19 in less duration of time. Therefore, the demand for single use systems has been propelled globally.
Market Segmentation
By Bioreactors
- Less than 1,000L
- 1,001-2,000L
- More than 2,000L
Based on bioreactors, the 1,000-2,000L segment acquired a prominent market share in the Global Single-Use System in the Biopharma manufacturing market in the historical years. This is expected to augment further as the global need for Biopharma products is rising, which would require the huge capacity of transportation of Biopharma products. However, due to the current technological limitations, the companies cater to their demand through lower capacity, which is less than 1000L.
Start-ups have proven to establish firm grounds for single use system technology which would facilitate the use of bioreactors less than 1000L even further. For instance: In 2020, the US-based startup New Horizon Biotech developed a patented fermentor system for flexible and cost-effective biopharmaceutical production. The system features a bag retention vessel, as well as agitation impellers and aerators, with mobility and flexibility in choosing capacity, ranging from 50 liters to 300 liters, for production.
By Product
- Single Use Sampling System
- Automated Sampling Solutions
- Off-line Single Use Sampling Solutions
- Reusable Manual Sampling Solutions
- Bioprocess Containers
- Containers
- Connectors
- Tubing
- Bioreactors
- Mixers
- Bottles
- Bottles
- Bottle Assemblies
- Transfer Sets
- Single Use Connectors
- Single Use Aseptic Connectors
- Disconnectors
- Single Use Aseptic Disconnectors
- Tubes & Assemblies
- Biowelder/Biosealer
- Others
Based on the product type, the single use sampling system acquired a considerable market share in the Global Single Use System in the Biopharma market during the historical period. The single use sampling systems are pre-irradiated with assemblies that ensure a sterilized fluid path for the cell culture sample to be examined. Its sampling system helps to reduce cross-contamination risks on a clinical scale. Therefore, the demand for single use sampling systems has increased for the manufacturing of biopharma products. Notably, various companies operating in the domain offer different types of connectors & disconnectors such as quick connectors & disconnectors, aseptic connectors & disconnectors, steam-to and steam-through connectors, etc., resulting in higher productivity.
With companies investing more in developing their biopharma manufacturing infrastructure through mergers & acquisitions (M&A), collaborations with the start-ups, and other strategic movements for expanding their regional capabilities, there has been an increasing R&D activity across various regions, including North America, Europe, and the Asia Pacific. Consequently, the demand for single use sampling systems is expected to experience considerable growth in the years to come
By Application
- Filtration
- Bioprocess Containers
- Bottles
- Storage (Media and Buffer, Freeze & Thaw)
- Bioprocess Containers
- Bottles
- Equipment
- Cell Culture
- Sampling System
- Bioreactor
- Bioprocess Container
- Mixing
- Mixers
- Purification
- Membrane Adsorbers
- Depth Filters
- Aseptic Filling
- Bioprocess Containers
- Bottles
- Transfer Units
By application type, the cell culture segment captured a notable market share during the historical period. The intensive requirement of the cell culture during the production of biological products & cell therapies such as vaccines, monoclonal antibodies, and other products has stimulated the demand for a single use system in biopharma.
Furthermore, with the introduction of biosimilars & generic biopharmaceutical drugs, the demand for cell culture has increased significantly. In addition, the increasing use of mammalian cell culture during upstream bioprocessing has augmented the demand for cell culture for better media. In addition, improving the reproducibility & consistency of the biopharma product & reducing the risk of contamination has driven the demand of the cell culture segment for the manufacturing of biopharma products.
By Bioprocessing
- Small-Scale
- Mid-Scale
- Large-Scale
Large-scale bioprocessing units are expected to gain a notable share in the global single use bio-pharma industry during 2017-21. With companies investing more in developing their biopharma manufacturing infrastructure through mergers & acquisitions (M&A) for expanding their regional capabilities, the demand for single use systems experienced considerable growth in recent years. The countries such as Germany, the US, China, and India, among others, have been notably investing in the R&D for biopharma manufacturing and are seen extending their long-term implications in the biopharma space.
By Modality
- Protein & Monoclonal Antibody (Mab)
- Cell Therapy
- Gene Therapy
- Conventional Vaccine
- mRNA
The cell & gene therapy market has gained momentum in recent years. The major factors contributing to the development of the cell and gene therapy market include the rising prevalence of chronic and infectious diseases, growing advancements in cancer drug discovery, increasing healthcare expenditure as well as growth in the research and development activities. These supportive factors resulted in an increase in production of cell and gene therapies globally and hence positively impacted the single use market in biopharma manufacturing. In addition, the presence of market players including, Biogen, Novartis, Catalent, Gilead Science, etc. laid the foundation for the development and production of cell and gene therapies, which in turn, accelerated the demand for single use system in biopharma manufacturing in near future
By Component
- Drug Substance
- Drug Product
Based on component, drug substance acquired significant market share in the global single use system in biopharma manufacturing during the 2017-21. As, drug substance, including active pharmaceutical ingredients (APIs) & excipients, is required at different stages of biopharma drug development, therefore, the demand for single use systems has been observed majorly in the clinical phase. Moreover, from the R&D initiation on a drug till the final approval from the healthcare regulatory body, there is a constant requirement of drug substance in pre-clinical, clinical, and different phases of trials conducted on a drug.
Regional Landscape
- North America
- South America
- Europe
- Middle East & Africa
- Asia Pacific
North America acquired a dominating market share in the Global Single Use Systems Market during the historical years. With the increase in demand for biologics & biosimilars in the region, single use bioprocessing products have been in high demand. Further, the biopharmaceutical companies in the region are heavily investing in R&D to explore the use of single-use systems as they offer many advantages, including minimal installation costs, low cross-contamination of products, increased energy efficiency, reduced usage of space, and quick installation. In 2019, Thermo Fisher invested around USD50 million in bio-production capabilities & capacity to manufacture single use bioprocessing systems for the application of liquid handling in the industry. Moreover, the company has made investments for capacity growth due to the high demand for Bioprocess Containers (BPCs) as BPCs and other fluid assemblers are the foundation of single use bioprocessing systems.
Recent Developments by Leading Companies
- In 2021, Pfizer Inc., invested USD800 million to open a clinical manufacturing facility in Durham, North Carolina. The facility supports the company’s continued investment in gene therapy research, development, and manufacturing.
- In July 2021, Cytiva and Pall Corporation, the subsidiaries of Danaher Corporation, announced an investment of USD1.5 billion to expand the biopharma manufacturing capacities in 13 sites globally.
Key Trends in the Market
- Rising Investments Towards Cells & Gene Therapy
The rapid evolution of the technology & research landscape in the field of Biopharma manufacturing has boosted the market growth of Biopharma products. Moreover, with the outbreak of the COVID-19, the use of single use bioprocessing systems has increased due to the high demand for vaccines around the world, which resulted in robust production & utilization of single use bioprocessing systems in cell & gene therapy. Moreover, the demand for a single use system in biopharma manufacturing for cell & gene therapy is expected to have exponential growth during 2022-27, due to the development of regenerative medicine. Besides, the rapidly growing regenerative medicine industry attributes primarily to the increasing number of patients diagnosed with chronic diseases, such as diabetes, Alzheimer's, cancer, renal failure, etc.
Furthermore, single-use technology offers drug manufacturers faster deliveries, due to less maintenance & sterilization of containers as compared to the stainless steel system. Additionally, it aids in the production of the cells & gene therapy, owing to its several advantages such as the low risk of cross-contamination during the mixing of samples. Hence, the growing investments in gene therapy & increasing focus to treat rare diseases are expected to escalate the market for single use systems during the forecast period.
Market Dynamics
Key Drivers
- Burgeoning Demand for Biopharmaceutical Products to Escalate the Market Growth
With the rising advancement in technology, the single-use bioprocessing system, has become one of the fastest-growing technology used during the manufacturing of biopharma products. It offers various benefits such as low initial investment, high flexibility as compared to the stainless steel systems, the enhanced production quality of biosimilars, etc. As a result, the demand for single use systems has been growing considerably globally.
In addition, as the number of chronic, infectious diseases, and rare diseases are increasing, the demand for bioprocessing products have also witnessed a notable uptick. Therefore, impelling the market growth of single-use bioprocessing systems in the biopharma space. Moreover, the large-scale biologic manufacturers have acquired SMEs to improve their vaccine production competencies so that they can meet the requirement of customers on a large scale.
Possible Restraint
- Qualifying Issues Related to Extractable & Leachable
Extractable & leachable are the chemical compounds that migrate from the single use system into model solvent & process solutions respectively from exaggerated temperature & time conditions. Hence, these are considered as a subset of extractable. However, during the production of pharmaceutical products, the purity of the final product is a necessity for better & more accurate results. Further, due to the extractable & leachable chemicals mixed with the end product, the quality of the biological product could deteriorate. Thus, biopharma products need to be assessed carefully while manufacturing & storing.
Moreover, single use bioprocessing products are often degraded by the containers due to leachable as the containers are made of plastic materials. Therefore, the extractable & leachable emerging from the components of single-use bioprocessing systems could hinder the market growth & could cause adverse effects on patients' health & product quality.
Key Questions Answered in the Market Research Report:
- What are the overall statistics or estimates (Overview, Market Size- By Value & Volume, Forecast Numbers, Market Segmentation, Market Shares) of the Global Single Use System in Biopharma Manufacturing Market?
- What are the country-wise industry size, growth drivers, and challenges?
- What are the key innovations, opportunities, current & future trends, and regulations in the Global Single Use System in Biopharma Manufacturing Market?
- Who are the key competitors, their key strengths & weaknesses, and how do they perform in the Global Single Use System in Biopharma Manufacturing Market based on the competitive benchmarking matrix?
- What are the key results derived from the surveys conducted during the Global Single Use System in Biopharma Manufacturing Market study?
Frequently Asked Questions
- Introduction
- Product Definition
- Research Process
- Assumptions
- Market Segmentation
- Executive Summary
- Customer Survey
- Respondents expect to see a 100% Single-Use facility in operation within 5 years
- Major reasons led to the adoption of single use system in biopharma manufacturing
- Problems faced by end users at the time of using single use system
- Trends in Bio-manufacturing
- Global Bio-Pharma Industry Overview
- Growth in Biopharmaceutical R&D
- Areas of innovation in biopharmaceutical manufacturing
- Impact of COVID-19 on Global Single Use System in Biopharma Manufacturing Market
- Global Single Use System in Biopharma Manufacturing Market Trends & Insights
- Global Single Use System in Biopharma Manufacturing Market Dynamics
- Growth Drivers
- Challenges
- Global Single Use System in Biopharma Manufacturing Market Policies, Regulations, Product Standards
- Global Single Use System in Biopharma Manufacturing Market- Supply Chain Analysis
- Global Single Use System in Biopharma Manufacturing Market Hotspot & Opportunities
- Global Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue (Including COVID-19 Impact)
- Market Revenue (Excluding COVID-19 Impact)
- Market Share & Analysis
- By Bioreactors
- Less than 1000L
- 1000-2000L
- More than 2000L
- By Product
- Single Use Sampling System
- Automated Sampling Solutions
- Off-line Single Use Sampling Solutions
- Reusable Manual Sampling Solutions
- Bioprocess Containers
- Containers
- Connectors
- Tubing
- Bioreactors
- Mixers
- Bottles
- Bottles
- Bottle Assemblies
- Transfer Sets
- Single Use Connectors
- Single Use Aseptic Connectors
- Disconnectors
- Single Use Aseptic Disconnectors
- Tubes & Assemblies
- Biowelder/Biosealer
- Others (Single use sensors, disposable filter cartridges)
- Single Use Sampling System
- By Application
- Filtration
- Disposable filter Cartridges
- Single use filtration bags
- Storage (Media and Buffer, Freeze & Thaw)
- Bioprocess Containers
- Bottles
- Equipment
- Cell Culture
- Single use Sampling system
- Bioreactor
- Bioprocess Containers
- Mixing
- Mixers
- Purification
- Membrane Adsorbers
- Depth Filters
- Aseptic Filling
- Bioprocess Containers
- Bottles
- Transfer Sets
- Filtration
- By Bioprocessing
- Small-scale
- Mid-scale
- Large-scale
- By Modality
- Protein & Monoclonal Antibody (Mab)
- Cell Therapy
- Gene Therapy
- Conventional Vaccine
- mRNA
- By Component
- Drug Substance
- Drug Product
- By Region
- North America
- South America
- Europe
- Middle East & Africa
- Asia-Pacific
- By Company
- Revenue Shares
- Strategic Factorial Indexing
- Competitor Placement in MarkNtel Quadrant
- By Bioreactors
- Market Size & Analysis
- North America Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Bioreactors
- By Product
- By Application
- By Bioprocessing
- By Modality
- By Component
- By Country
- The US
- Canada
- The US Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Canada Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Market Size & Analysis
- South America Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Bioreactors
- By Product
- By Application
- By Bioprocessing
- By Modality
- By Component
- By Country
- Brazil
- Rest of South America
- Brazil Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Market Size & Analysis
- Europe Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Bioreactors
- By Product
- By Application
- By Bioprocessing
- By Modality
- By Component
- By Country
- Germany
- The UK
- France
- Rest of Europe
- Germany Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- The UK Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- France Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Rest of Europe Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Market Size & Analysis
- Middle East & Africa Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Bioreactors
- By Product
- By Application
- By Bioprocessing
- By Modality
- By Component
- By Country
- Saudi Arabia
- South Africa
- Rest of MEA
- Saudi Arabia Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- South Africa Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Rest of MEA Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Market Size & Analysis
- Asia-Pacific Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Bioreactors
- By Product
- By Application
- By Bioprocessing
- By Modality
- By Component
- By Country
- China
- India
- Japan
- South Korea
- Rest of Asia-Pacific
- China Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- India Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Japan Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- South Korea Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Rest of Asia-Pacific Single Use System in Biopharma Manufacturing Market Outlook, 2017-2027F
- Market Size & Analysis
- Market Revenue
- Market Share & Analysis
- By Product
- By Application
- Market Size & Analysis
- Market Size & Analysis
- Global Single Use System in Biopharma Manufacturing Market Key Strategic Imperatives for Success & Growth
- Competition Outlook
- Competition Matrix
- Product Portfolio
- Target Markets
- Research & Development
- Strategic Alliances
- Strategic Initiatives
- Company Profiles of top 13 companies (Business Description, Product Segments, Business Segments, Financials, Strategic Alliances/ Partnerships, Future Plans)
- Sartorius AG (Sartorius Stedim Biotech)
- Entegris
- Pall Corporation
- Eppendorf
- Corning Incorporated
- ThermoFisher Scientific
- PBS Biotech Inc.
- Lonza
- Merck KGaA
- Danaher Corporation
- Kühner AG
- Rentschler Biopharma SE
- Getinge AB
- Others
- Competition Matrix
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
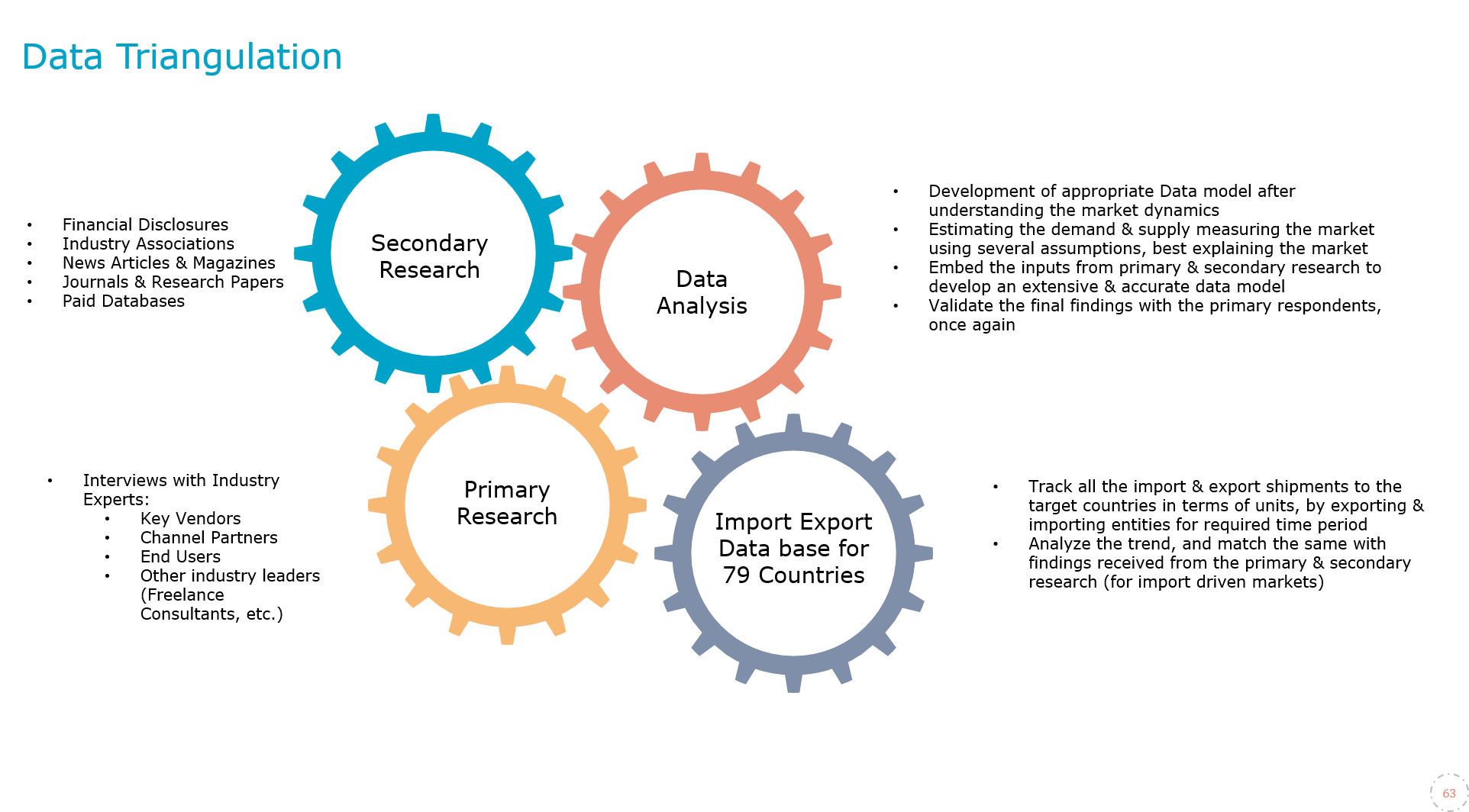
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









