
North America Virtual Clinical Trials Market Analysis, 2020
By Type (Interventional Trials, Expanded Access Trials, Observational Trials), By Application (Cardiovascular Disease, Oncology, Others), By Country (The US, Canada, Mexico), By Co...mpany (ICON Plc, Clinical Ink, Inc., Science 37, Inc., Medable, Inc., Medidata Solutions, Signant Health (CRF Health), PRA Health Sciences, Oracle Corporation, Covance, Inc., Parexel International etc.) Read more
- Healthcare
- Oct 2020
- Pages 165
- Report Format: PDF, Excel, PPT
Virtual clinical trials, also known as remote or decentralized trials, is a method to conduct clinical research with the use of digital health technologies such as apps and electronic monitoring devices, along with online social engagement platform.
North America Virtual Clinical Trials Market Analysis, 2020 research report depicts a deep-dive market analysis of statistics of North America Virtual Clinical Trials market which consists of regional and country-wise market size, market forecast, CAGR market segmentation, market shares of diverse regions and countries, market share of various end-users, applications, product type, technologies, competitive benchmarking, etc.
Market Opportunities
According to MarkNtel Advisors’ research report titled “North America Virtual Clinical Trials Market Analysis, 2020”, the North America Virtual Clinical Trials (VCTs) market is anticipated to grow at a considerable CAGR of during 2020-25F. The booming aging population, growing incorporation of patient-facing technology such as tablets, wearable sensors in the virtual clinical trials, and increasing inclination to conduct safe and effective research at a lower cost remotely is surging virtual clinical trials leverage to incline toward the use of telehealth technologies like remote patient monitoring and wearable mHealth devices. Thus, this is projected to drive the growth of the market in the coming years. Moreover, the COVID-19 pandemic has had an optimistic impact on the growth of the virtual clinical trial market due to the rise in the adoption of VCTs on account of extensive demand for vaccines and also due to lockdown restriction which has surged the rate of patient’s inclination toward video conference calls, online chat, and apps for communicating with healthcare providers.
Market Segmentation
Oncology Segment Acquired the Majority Market Share
Based on the Application, the Oncology segment acquired the largest market share in the North America virtual clinical trial market in 2019. The growth of the segment is attributed to the burgeoning number of cancer cases, increasing the geriatric population, and surging investment in the R&D activities of oncology drugs. According to Canadian Cancer Statistics, 225,800 new cases of cancer are expected to be diagnosed in Canada in 2020. Moreover, cardiovascular disease is also anticipated grow at the fastest rate on account of the increasing prevalence of heart failure, which would further promote the growth of the Virtual Clinical Trials market in North America in the coming years as stated in the MarkNtel Advisors’ research report “North America Virtual Clinical Trials Market Analysis, 2020”.

Competitive Landscape
According to MarkNtel Advisors, the key players with a considerable market share in the North America Virtual Clinical Trials market include ICON Plc, Clinical Ink, Inc., Science 37, Inc., Medable, Inc., Medidata Solutions, Signant Health (CRF Health), PRA Health Sciences, Oracle Corporation, Covance, Inc., Parexel International, etc.
Key Questions Answered in the Market Research Report:
- What are the key overall market statistics or market estimates (Market Overview, Market Size- By Value, Forecast Numbers, Market Segmentation, Market Shares) of the North America Virtual Clinical Trials Market?
- What is the region-wise industry size, growth drivers, and challenges key market trends?
- What are the key innovations, technology upgrades, opportunities, regulations in the North America Virtual Clinical Trials Market?
- Who are the key competitors or market players and how they perform in North America Virtual Clinical Trials Market based on the competitive benchmarking matrix?
- What are the key results derived from the market surveys conducted during the course of the North America Virtual Clinical Trials Market study?
Market Outlook, Segmentation, and Statistics
- Impact of COVID-19
- Market Size & Analysis
- By Revenues (USD Million)
- Market Share & Analysis
- Type
- Interventional Trials
- Expanded Access Trials
- Observational Trials
- By Application
- Cardiovascular Disease
- Oncology
- Others
- By Region
- The US Virtual Clinical Trials Market Outlook, 2015-2025F (USD Million)
- Canada Virtual Clinical Trials Market Outlook, 2015-2025F (USD Million)
- Mexico Virtual Clinical Trials Market Outlook, 2015-2025F (USD Million)
- By Company
- Revenue Shares
- Strategic Factorial Indexing
- Competitor Placement in MarkNtel Quadrant
- Type
- North America Virtual Clinical Trials Market Policies, Regulations, Product Standard
- North America Virtual Clinical Trials Market Hotspots & Opportunities
- Competition Outlook
- Competitor Wise Growth Strategies
- Company Profiles
Frequently Asked Questions:
Q. What is the historical year, base year and forecast year considered in the research report on North America Virtual Clinical Trials Market?
A. The historical data has been provided since 2015, while the base year is 2019 and the data is forecast up-to 2025.
Q. What are the units or denomination for measuring market value and volume in the report?
A. The market size/industry size or the market value is measured in terms of USD Million.
Q. What would be the growth rate or CAGR of North America Virtual Clinical Trials Market during 2020-25?
A. The growth of North America Virtual Clinical Trials Market is forecast to be grow at a considerable rate during 2020-25.
Q. Who are the key competitors or players operating in North America Virtual Clinical Trials Market?
A. ICON Plc, Clinical Ink, Inc., Science 37, Inc., Medable, Inc., Medidata Solutions, Signant Health (CRF Health), PRA Health Sciences, Oracle Corporation, Covance, Inc., Parexel International etc., are few of the leading players in the North America Virtual Clinical Trials Market.
Q. Which application segment would emerge as an opportunity area for players in North America Virtual Clinical Trials Market?
A. Oncology segment is expected to attain the highest CAGR during the forecast period and is expected maintain its significant market share.
Q. Which country would emerge as an opportunity area for players in North America Virtual Clinical Trials Market?
A. The US market continue to grow at a highest CAGR, presenting immense opportunities for market players of North America Virtual Clinical Trials Market.
Frequently Asked Questions
- Introduction
- Product Definition
- Research Process
- Assumptions
- Market Segmentation
- Preface
- Executive Summary
- Impact of COVID-19 on North America Virtual Clinical Trials Market
- North America Virtual Clinical Trials Market Analysis, 2015-2025F
- Market Size & Analysis
- Revenues (in USD Million)
- Market Share & Analysis
- By Type
- Interventional Trials
- Expanded Access Trials
- Observational Trials
- By Application
- Cardiovascular Disease
- Oncology
- Others
- By Region
- The US
- Canada
- Mexico
- By Competitors
- Competition Characteristics
- Market Share & Analysis
- Competitive Metrix
- By Type
- Market Size & Analysis
- The US Virtual Clinical Trials Market Analysis, 2015-2025F
- Market Size & Analysis
- Revenues (in USD Million)
- Market Share & Analysis
- By Type
- By Application
- Market Size & Analysis
- Canada Virtual Clinical Trials Market Analysis, 2015-2025F
- Market Size & Analysis
- Revenues (in USD Million)
- Market Share & Analysis
- By Type
- By Application
- Market Size & Analysis
- Mexico Virtual Clinical Trials Market Analysis, 2015-2025F
- Market Size & Analysis
- Revenues (in USD Million)
- Market Share & Analysis
- By Type
- By Application
- Market Size & Analysis
- North America Virtual Clinical Trials Market Regulations, Policies, Product Benchmarks
- North America Virtual Clinical Trials Market Trends & Insights
- North America Virtual Clinical Trials Market Dynamics
- Drivers
- Challenges
- North America Virtual Clinical Trials Market Hotspot & Opportunities
- North America Virtual Clinical Trials Market, Competition Outlook
- Competition Matrix
- Target Markets
- Target Applications
- Research & Development
- Collaborations & Strategic Alliances
- Key Business Expansion Initiatives
- Business Restructuring- Mergers, Acquisitions, JVs
- Strategic Initiatives
- Company Profiles (Business Description, Product Offering, Strategic Alliances or Partnerships, etc.)
- ICON Plc
- Clinical Ink, Inc.
- Science 37, Inc.
- Medable, Inc.
- Medidata Solutions
- Signant Health (CRF Health)
- PRA Health Sciences
- Oracle Corporation
- Covance, Inc.
- Parexel International
- Competition Matrix
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
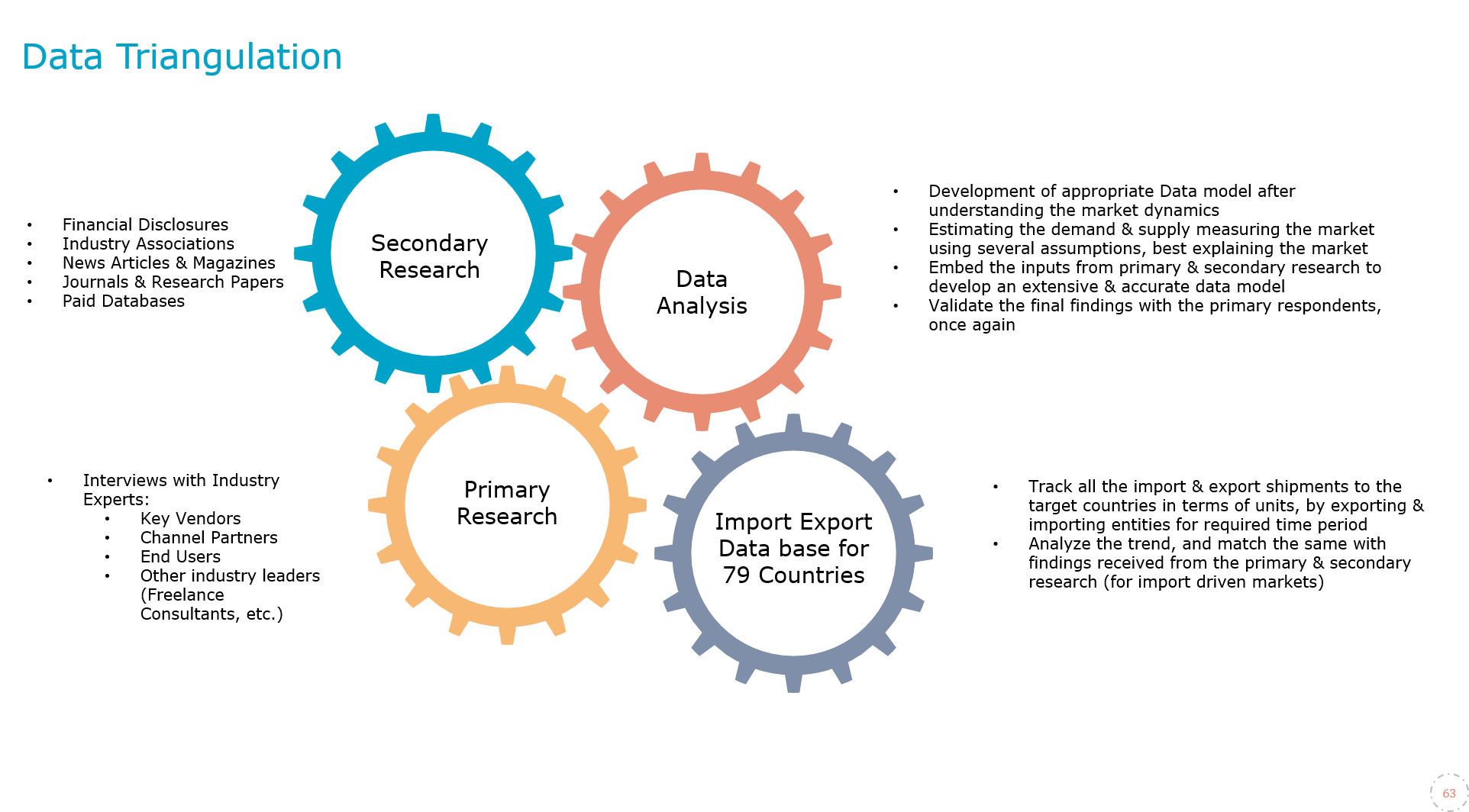
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









