
Nigeria In-Vitro Diagnostics (IVD) Market Research Report: Forecast (2026-2032)
Nigeria In-Vitro Diagnostics (IVD) Market - By Product Type (Reagents & Kits, Instruments, Consumables, Software & Services) By Technology– (Immunoassays, Molecular Diagno...stics, Clinical Chemistry, Microbiological Diagnostics, Hematologic Diagnostics, Coagulation, Others), By Distribution Channel (Direct Tender, Retail Pharmacy Chains, Online Distribution Platforms, Third-Party Distributors & Wholesalers), By End User (Hospitals & Clinics, Diagnostic Laboratories, Blood Banks, Point-of-Care Testing Centers, Research Institutes & Academic Settings, Others (e.g., mobile clinics, community health centers), By Application (Infectious Diseases, Malaria, HIV/AIDS, Tuberculosis, Hepatitis B & C, Lassa Fever, Others (Diphtheria), Chronic Diseases, Diabetes, Cardiovascular Diseases, Oncology, Nephrology, Others), and others Read more
- Healthcare
- Dec 2025
- Pages 135
- Report Format: PDF, Excel, PPT
Nigeria In-Vitro Diagnostics (IVD) Market
Projected 9.89% CAGR from 2026 to 2032
Study Period
2026-2032
Market Size (2025)
USD 41.29 Million
Market Size (2032)
USD 79.92 Million
Base Year
2025
Projected CAGR
9.89%
Leading Segments
By Product Type: Reagents & Kits
Nigeria In-Vitro Diagnostics (IVD) Market Size: Forecast (2026-2032)
The Nigeria In-Vitro Diagnostics (IVD) Market size is valued at around USD41.29 million in 2025 and is projected to reach USD79.92 million by 2032. Along with this, the market is estimated to grow at a CAGR of around 9.89% during the forecast period, i.e., 2026-32.
Nigeria In-Vitro Diagnostics (IVD) Market Outlook:
Nigeria’s IVD Market is strengthening rapidly as the country responds to some of the most severe disease burdens in Africa. Nigeria accounts for over a quarter of global malaria cases (WHO), faces hundreds of thousands of tuberculosis infections every year, and continues to manage widespread HIV, Lassa fever, and diphtheria outbreaks, with significant Lassa fever activity reported nationwide in 2025 and diphtheria affecting thousands of citizens across multiple states since 2023 (NCDC, WHO). These high and persistent burdens create continuous demand for timely diagnostics across hospitals, clinics, and public-health programs.
Additionally, government programmes are also widening access to critical diagnostic services. Under Nigeria’s HIV programme, millions of people are screened annually for HIV, while the National Blood Service Commission (NBSC) conducts safety testing for over 500,000 donated blood units every year, each requiring mandatory screening for HIV, HBV, HCV, and syphilis.
Likewise, Nigeria’s Malaria Elimination Plan 2021–2025 aims to reduce malaria prevalence below 10%, prompting nationwide scale-up of rapid tests, microscopy, and surveillance diagnostics. This commitment strengthens demand for IVD kits and supports continuous investment in laboratory and community-level testing. These initiatives generate great, predictable demand for rapid tests, ELISA kits, molecular reagents, and laboratory consumables.
Moreover, investments are visible on the infrastructure side as well. The Nigeria Sovereign Investment Authority (NSIA), through its Healthcare Development and Investment Company, is funding 23 state-of-the-art diagnostic and oncology centres across the country. The Federal Government’s Basic Healthcare Provision Fund also allocated billions of naira to strengthen primary-care diagnostics, ensuring rural facilities gain access to essential testing equipment.
Nigeria In-Vitro Diagnostics (IVD) Market Recent Developments:
- 2025: Codix Bio opened Nigeria's first large-scale rapid diagnostic test (RDT) manufacturing plant in Ogun State. The facility is designed to produce millions of HIV, malaria, hepatitis, and other essential test kits, strengthening local diagnostic capacity and reducing reliance on imported supplies.
- 2025: Roche Diagnostics established a new legal entity in Nigeria, strengthening its direct presence and commitment to expanding diagnostic access. The company aims to enhance the availability of molecular and tissue diagnostics, along with near-patient testing solutions across the country.
Nigeria In-Vitro Diagnostics (IVD) Market Scope:
| Category | Segments |
|---|---|
| By Product Type | Reagents & Kits, Instruments, Consumables, Software & Services) |
| By Technology | Immunoassays, Molecular Diagnostics, Clinical Chemistry, Microbiological Diagnostics, Hematologic Diagnostics, Coagulation, Others), |
| By Distribution Channel | Direct Tender, Retail Pharmacy Chains, Online Distribution Platforms, Third-Party Distributors & Wholesalers), |
| By End User | Hospitals & Clinics, Diagnostic Laboratories, Blood Banks, Point-of-Care Testing Centers, Research Institutes & Academic Settings, Others (e.g., mobile clinics, community health centers), |
| By Application | Infectious Diseases, Malaria, HIV/AIDS, Tuberculosis, Hepatitis B & C, Lassa Fever, Others (Diphtheria), Chronic Diseases, Diabetes, Cardiovascular Diseases, Oncology, Nephrology, Others), and others |
Nigeria In-Vitro Diagnostics (IVD) Market Drivers:
Rising Cases of Infectious Diseases Accelerating Diagnostic Demand
Nigeria’s heavy infectious-disease burden remains one of the strongest forces driving growth in this market, as widespread and recurring illnesses create continuous demand for reagents, rapid test kits, and laboratory consumables. According to the World Health Organization, Nigeria accounts for about 27% of global malaria cases, leading to tens of millions of suspected cases each year, each requiring rapid testing or microscopy.
Beyond malaria, the prevalence of other infectious diseases reinforces high diagnostic volume. For instance, Nigeria recorded an estimated 460,000 tuberculosis (TB) cases in 2023, placing it among the world’s high-burden countries. In addition, the Nigeria Centre for Disease Control reported 758 confirmed Lassa fever cases with 143 deaths in 2025, highlighting a significant outbreak across 18 states. Nigeria also experienced one of its largest diphtheria outbreaks in recent history, with 557 confirmed cases across 21 states and the FCT since 2023 (WHO). These outbreaks require rapid testing, PCR confirmation, and repeated surveillance, increasing the use of molecular and serology-based diagnostics.
Moreover, HIV testing is also a major contributor to the country’s diagnostic workload. National HIV estimates indicate about 1.9 million people living with HIV in Nigeria as of 2025, and PEPFAR-supported programmes conduct millions of HIV screening and viral-load tests annually. This steady activity drives significant consumption of immunoassays, PCR reagents, and quality-control materials, particularly in high-volume hospitals and clinics.
Nigeria In-Vitro Diagnostics (IVD) Market Trends:
Integration of Artificial Intelligence in IVD Devices
Artificial intelligence (AI) is becoming a noticeable trend in Nigeria’s In-Vitro Diagnostics (IVD) Market as healthcare providers begin adopting digital tools to improve test accuracy and speed. For instance, in 2025, the deployment of BloodGPT, an AI-powered diagnostic-interpretation platform introduced in Nigeria through a partnership between Envisionit Deep AI and Wazima Health. This system helps laboratories interpret blood-test results automatically, highlight abnormal values, and reduce review time for clinicians, marking one of the first documented AI-assisted diagnostic tools used in Nigerian laboratory settings.
Beyond this deployment, several Nigerian teaching hospitals and private laboratories are exploring AI for automated quality control, digital workflow management, and improved result validation. Moreover, African Laboratory Medicine studies (2024) report increasing interest in AI tools to support laboratories facing high testing volumes and limited specialist staff, conditions common in Nigeria, which are actively transforming this market.
Nigeria In-Vitro Diagnostics (IVD) Market Challenges:
High Import Dependence and Weak Laboratory Infrastructure
Nigeria’s In-Vitro Diagnostics (IVD) Market faces a major challenge, including heavy reliance on imported diagnostic supplies and persistent gaps in laboratory infrastructure. For instance, the country imported over USD 4.4 million worth of medical diagnostic test kits in 2023, and malaria diagnostic-kit imports reached approximately USD 12.4 million in the same year. This level of reliance exposes the healthcare system to foreign-exchange fluctuations, international supply disruptions, and higher procurement costs, especially during periods of global demand surges.
At the facility level, many hospitals and clinics struggle with inadequate laboratory capacity. National health-facility assessments highlight issues such as unstable electricity, limited cold-chain storage, outdated or non-functional analyzers, and shortages of trained laboratory personnel.
These constraints limit the ability of many primary and secondary healthcare facilities to perform reliable chemistry, hematology, microbiology, or molecular tests. As a result, advanced diagnostics remain concentrated in tertiary hospitals such as LUTH, UCH Ibadan, AKTH, and National Hospital Abuja, thus hindering the market growth.
Nigeria In-Vitro Diagnostics (IVD) Market (2026-32) Segmentation Analysis:
The Nigeria In-Vitro Diagnostics (IVD) Market Report and Forecast 2026-2032 offers a detailed analysis of the market based on the following segments:
Based on Product Type
- Reagents & Kits
- Instruments
- Consumables
- Software & Services
Reagents and test kits hold a dominant position in Nigeria’s In-Vitro Diagnostics (IVD) Market because they are required for the country’s highest-volume medical tests, particularly in infectious-disease screening and routine clinical diagnostics. National programs for HIV, malaria, hepatitis, blood safety, and antenatal care depend heavily on rapid tests and biochemical reagents, which must be replenished continuously. In contrast, instruments and software are purchased infrequently and do not generate the same recurring demand.
The strength of this segment was further reinforced in 2025 with the commissioning of Codix Bio’s large-scale diagnostic-kit manufacturing plant in Ogun State, which has an initial production capacity of 147 million test kits per year. This facility marks a major step toward improving local availability of essential reagents and reducing reliance on imported kits. Together, these factors confirm reagents and test kits as the leading and most indispensable component of Nigeria’s IVD Market.
Based on End User
- Hospitals & Clinics
- Diagnostic Laboratories
- Blood Banks
- Point-of-Care Testing Centers
- Research Institutes & Academic Settings
- Others (mobile clinics, community health centers, etc.)
Hospitals and clinics dominate Nigeria’s in-vitro diagnostics (IVD) industry because they are the only facilities with the infrastructure, staff, and capacity to handle large-scale and complex testing. Nigeria has over 40,000 healthcare facilities, and most are hospitals or clinics that people visit first for care. These facilities also run major national health programs. Nigeria encounters numerous malaria cases, and most malaria tests, microscopy, and rapid tests are performed in hospitals and clinics. Hospitals also support HIV Testing Services, which screen millions of Nigerians every year.
Their dominance is further strengthened by better laboratory equipment and trained professionals. Teaching and specialist hospitals have advanced chemistry analyzers, hematology systems, microbiology labs, and sometimes PCR platforms, which smaller centers often lack. Many hospitals also have more reliable electricity and cold-chain capability, making it possible to run tests requiring controlled conditions.
Leading Players of the Nigeria In-Vitro Diagnostics (IVD) Market:
-
Roche Diagnostics (Established 1896)
Roche Diagnostics is one of the most influential global healthcare companies offering advanced solutions across immunoassays, clinical chemistry, hematology, and molecular diagnostics. In Nigeria, the company plays a crucial role in strengthening disease detection for conditions such as HIV, hepatitis, tuberculosis, and non-communicable diseases. Its automated analyzers and PCR systems support hospitals, public labs, and disease-control initiatives by enabling high-accuracy and scalable testing. Roche’s long-standing investment in diagnostic innovation continues to elevate laboratory standards across the country.
-
Abbott Laboratories (Established 1888)
Abbott brings a strong portfolio of rapid tests, point-of-care devices, and core laboratory systems that are widely deployed across Nigeria’s public and private healthcare ecosystem. The company is especially recognized for its HIV and malaria rapid diagnostic kits, which support national screening and treatment programs. Abbott’s focus on accessible diagnostics, combined with its global expertise in infectious-disease testing, makes it a key enabler of early detection and improved patient outcomes across Nigeria.
-
Bio-Rad Laboratories (Established 1952)
Bio-Rad is a globally trusted supplier of quality-control products, molecular diagnostics kits, and immunology testing solutions. Nigerian laboratories rely on Bio-Rad’s QC materials to ensure testing accuracy and regulatory compliance. The company’s instruments and consumables help improve the reliability of diagnostics in blood banks, research laboratories, and clinical facilities, reinforcing quality assurance across the national lab ecosystem.
Siemens Healthineers, Danaher Corporation, Thermo Fisher Scientific, Cepheid, Qiagen N.V., Codix Pharma Ltd., and Others are the key players in the Nigerian In-Vitro Diagnostics (IVD) Market.
Nigeria In-Vitro Diagnostics (IVD) Market (2026-32): Regional Projection
The southern region, dominated by Lagos, is leading this market. This is due to its unmatched concentration of healthcare infrastructure, population density, and diagnostic capacity. The Lagos State Ministry of Health reports that the state hosts more than 3,00 registered public health facilities, the highest number in Nigeria, alongside major public institutions such as Lagos University Teaching Hospital (LUTH) and Lagos State University Teaching Hospital (LASUTH). This extensive network drives the largest diagnostic testing volume in the region.
For instance, Lagos is home to Nigeria’s major ISO 15189-accredited laboratories, strengthening trust in laboratory quality and attracting higher testing demand. Additionally, Lagos accounts for the country’s largest urban population, projecting over 17 million residents, intensifying the need for high-capacity diagnostic services.
Several key diagnostic companies have also chosen Lagos as their operational base. The presence of multinational distributors for Abbott, Siemens Healthineers, Thermo Fisher, and Cepheid further reinforces Lagos as the primary commercial and technological hub for the IVD market in southern Nigeria.
Gain a Competitive Edge with Our Nigeria In-Vitro Diagnostics (IVD) Market Report
- Nigeria In-Vitro Diagnostics (IVD) Market Report by MarkNtel Advisors provides a detailed & thorough analysis of market size & share, growth rate, competitive landscape, and key players. This comprehensive analysis helps businesses gain a holistic understanding of the market dynamics & make informed decisions.
- This report also highlights current market trends & future projections, allowing businesses to identify emerging opportunities & potential challenges. By understanding market forecasts, companies can align their strategies & stay ahead of the competition.
- Nigeria In-Vitro Diagnostics (IVD) Market Report aids in assessing & mitigating risks associated with entering or operating in the market. By understanding market dynamics, regulatory frameworks, and potential challenges, businesses can develop strategies to minimize risks & optimize their operations.
*Reports Delivery Format - Market research studies from MarkNtel Advisors are offered in PDF, Excel and PowerPoint formats. Within 24 hours of the payment being successfully received, the report will be sent to your email address.
Frequently Asked Questions
- Market Segmentation
- Introduction
- Product Definition
- Research Process
- Assumptions
- Executive Summary
- Nigeria In-Vitro Diagnostics (IVD) Market Policies, Regulations, and Product Standards
- Nigeria In-Vitro Diagnostics (IVD) Market Supply Chain Analysis
- Nigeria In-Vitro Diagnostics (IVD) Market Trends & Developments
- Nigeria In-Vitro Diagnostics (IVD) Market Dynamics
- Growth Drivers
- Challenges
- Trends
- Opportunities
- Nigeria In-Vitro Diagnostics (IVD) Market Hotspot & Opportunities
- Nigeria In-Vitro Diagnostics (IVD) Market Pricing Analysis
- Nigeria In-Vitro Diagnostics (IVD) Market Strategic Insights
- Nigeria In-Vitro Diagnostics (IVD) Market Outlook, 2022-2032
- Market Size & Outlook
- By Revenues (USD Million)
- Market Share & Outlook
- By Product Type– Market Size & Forecast 2022-2032, USD Million
- Reagents & Kits
- Instruments
- Consumables
- Software & Services
- By Technology– Market Size & Forecast 2022-2032, USD Million
- Immunoassays
- Molecular Diagnostics
- Clinical Chemistry
- Microbiological Diagnostics
- Hematologic Diagnostics
- Coagulation
- Others
- By Distribution Channel– Market Size & Forecast 2022-2032, USD Million
- Direct Tender
- Retail Pharmacy Chains
- Online Distribution Platforms
- Third-Party Distributors & Wholesalers
- By End User– Market Size & Forecast 2022-2032, USD Million
- Hospitals & Clinics
- Diagnostic Laboratories
- Blood Banks
- Point-of-Care Testing Centers
- Research Institutes & Academic Settings
- Others (e.g., mobile clinics, community health centers)
- By Application– Market Size & Forecast 2022-2032, USD Million
- Infectious Diseases
- Malaria
- HIV/AIDS
- Tuberculosis
- Hepatitis B & C
- Lassa Fever
- Others (Diphtheria)
- Chronic Diseases
- Diabetes
- Cardiovascular Diseases
- Oncology
- Nephrology
- Others
- Infectious Diseases
- By Region
- North
- South
- East
- West
- Central
- By Product Type– Market Size & Forecast 2022-2032, USD Million
- Market Size & Outlook
- Nigeria Reagents & Kits In-Vitro Diagnostics (IVD) Market Outlook, 2022-2032
- Market Size & Outlook
- By Revenues (USD Million)
- Market Share & Outlook
- By Technology– Market Size & Forecast 2022-2032, USD Million
- By Distribution Channel– Market Size & Forecast 2022-2032, USD Million
- By End-Users– Market Size & Forecast 2022-2032, USD Million
- By Application– Market Size & Forecast 2022-2032, USD Million
- Market Size & Outlook
- Nigeria Instruments In-Vitro Diagnostics (IVD) Market Outlook, 2022-2032
- Market Size & Outlook
- By Revenues (USD Million)
- Market Share & Outlook
- By Technology– Market Size & Forecast 2022-2032, USD Million
- By Distribution Channel– Market Size & Forecast 2022-2032, USD Million
- By End-Users– Market Size & Forecast 2022-2032, USD Million
- By Application– Market Size & Forecast 2022-2032, USD Million
- Market Size & Outlook
- Nigeria Consumables In-Vitro Diagnostics (IVD) Market Outlook, 2022-2032
- Market Size & Outlook
- By Revenues (USD Million)
- Market Share & Outlook
- By Technology– Market Size & Forecast 2022-2032, USD Million
- By Distribution Channel– Market Size & Forecast 2022-2032, USD Million
- By End-Users– Market Size & Forecast 2022-2032, USD Million
- By Application– Market Size & Forecast 2022-2032, USD Million
- Market Size & Outlook
- Nigeria Software & Services In-Vitro Diagnostics (IVD) Market Outlook, 2022-2032
- Market Size & Outlook
- By Revenues (USD Million)
- Market Share & Outlook
- By Technology– Market Size & Forecast 2022-2032, USD Million
- By Distribution Channel– Market Size & Forecast 2022-2032, USD Million
- By End-Users– Market Size & Forecast 2022-2032, USD Million
- By Application– Market Size & Forecast 2022-2032, USD Million
- Market Size & Outlook
- Nigeria In-Vitro Diagnostics (IVD) Market Key Strategic Imperatives for Success & Growth
- Competition Outlook
- Company Profiles
- Roche Diagnostics
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Abbott Laboratories
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Siemens Healthineers
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Danaher Corporation
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Bio-Rad Laboratories
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Thermo Fisher Scientific
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Cepheid
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Qiagen N.V.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Codix Pharma Ltd.
- Business Description
- Product Portfolio
- Strategic Alliances or Partnerships
- Recent Developments
- Financial Details
- Others
- Others
- Roche Diagnostics
- Company Profiles
- Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
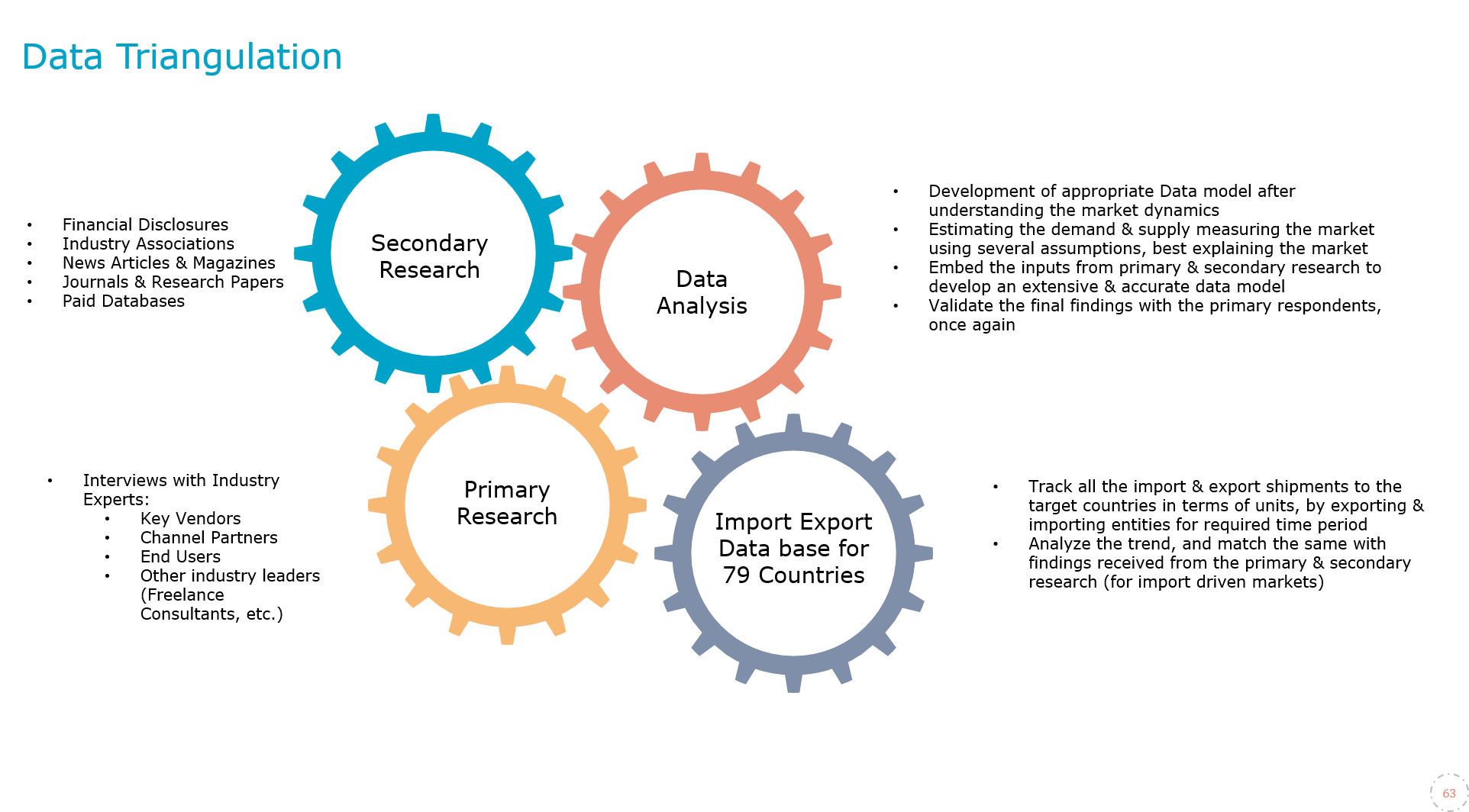
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









