
Global Electronic Trial Master Files Market Analysis, 2020
Global Electronic Trial Master File (eTMF) Systems Market Analysis,2020, By Component (Service and Software), By Mode of Delivery (Cloud Based and On-Premise), By End User (Contrac...t Research firms and Pharmaceutical and Biotechnology Companies), By Region (North America, Latin America, Europe, Middle East and Africa and Asia-Pacific), By Country (United States, Canada, Mexico, Brazil, Germany, United Kingdom, Spain, France, GCC, South Africa, China, India, Japan, Australia), By Company Read more
- Healthcare
- Jan 2020
- Pages 229
- Report Format: PDF, Excel, PPT
The rising clinical trials and quick adoption of electronic trail master file systems is positively driving the eTMF systems market. The systems are used for accessing documents from remote locations providing safety and secure transfer of data. Rising work efficiency, cost effectiveness, improving quality of data and saving time are some major factors boosting the demand for electronic trial master file systems. Moreover, manufacturers are merging and acquiring businesses to gain the competitive edge and stay in the competition by developing new technologies.
| Report Coverage | Details |
| Study Period | Historical Data: 2015-19 |
| Base Year: 2019 | |
| Forecast Period: 2020-25 | |
| Regions Covered | North America |
| Europe | |
| Asia-Pacific | |
| South America | |
| Middle East & Africa | |
| Unit Denominations | USD Million/Billion |
According to the MarkNtel Advisors’ research report titled “Global Electronic Trial Master File (eTMF) Systems Market Analysis, 2020”, the CAGR of the market is expected to be around 12% during 2020-25.
Burgeoning Clinical Trials Helping to Boost the Demand for Electronic Trial Master File Systems
Due to rising clinical trials and huge research and development budgets by biotechnology and pharmaceutical companies, the demand for electronic trail master file systems market is witnessing a growth and is anticipated to further grow during 2020-25.
North America dominated the market by the largest share in 2019 with the increasing use of cloud technology and quick adoption of new technology. Asia-Pacific is emerging as a lucrative market with the improving healthcare infrastructure. Moreover, China and India are witnessing an upsurge with the increasing establishment of pharmaceutical companies and sale of good quality medicines at lower prices.
Cloud-based segment acquired the largest market share in 2019 owing to the increasing adoption of cloud-based solutions worldwide. Cloud-based solutions offer flexibility and affordability is expected to further driving the market of eTMF systems during 2020-25.
However, data privacy issues and lack of skilled professionals are curbing the growth of electronic trial master file systems.
According to the MarkNtel Advisors, the leading players are Oracle Corporation, Veeva Systems, Mastercontrol, Transperfect, Sureclinical, Lab Corp, Mayo Clinic, BIOVIA Corp., Aurea Software, Wingspan Technology among others.
Montrium’s Electronic trail master file would be used by Altasciences, a biotech and pharama contract research organisation. This eTMF system is used for the centralized interface and better management of the clinical trials.
“Global Electronic Trial Master File (eTMF) Systems Market Analysis, 2020”, provides comprehensive qualitative and quantitative insights on the industry potential, key factors such as trends, drivers, hotspots and growth opportunities and challenges available for electronic trail master file systems providers across the globe. Moreover, the report also encompasses the key leading players in the industry, along with competitive benchmarking and competition matrix and company profiling.
Key Questions Answered in the Market Research Report
- What are the key market statistics (Market Overview, Market Size, Forecast, CAGR, Market Segmentation, Market Shares) of Global Electronic Trial Master Files Market?
- What are the key market or technology trends in Global Electronic Trial Master Files Market?
- What are the significant innovations, technology upgrades, opportunities, regulations, growth drivers and challenges in the Global Electronic Trial Master Files Market?
- Who are the key competitors or market players and how they perform in global competitive benchmarking matrix?
- What are the key results derived from the market surveys conducted during the course of Global Electronic Trial Master Files Market study?
Market Outlook, Segmentation and Statistics
- Market Size & Forecast
- By Revenues (USD Billion)
- By Volume (Units Sold)
- Market Share & Forecast
- By Component
- Services
- Software
- By Mode of Delivery
- Cloud-Based
- On-Premise
- By End User
- Pharmaceutical and Biotechnology Companies
- Contract Research Firms
- By Region
- North America
- United State
- Canada
- Latin America
- Brazil
- Mexico
- Europe
- United Kingdom
- Spain
- France
- Germany
- Middle East & Africa
- South Africa
- GCC
- Turkey
- Asia-Pacific
- China
- India
- Japan
- Australia
- North America
- By Company
- Revenue Shares
- Strategic Factorial Indexing
- Competitor Placement in MarkNtel Quadrant
- By Component
- North America Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F (USD Million)
- Latin America Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F (USD Million)
- Europe Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F (USD Million)
- Middle East and Africa Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F (USD Million)
- Asia-Pacific Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F (USD Million)
- Global Electronic Trial Master File (eTMF) Systems Market Policies, Regulations, Product Standards
- Global Electronic Trial Master File (eTMF) Systems Market Hotspot & Opportunities
- Global Electronic Trial Master File (eTMF) Systems Market Key Strategic Imperatives for Success & Growth
- Global Competition Outlook
- Competition Matrix
- Company Profile
Frequently Asked Questions
Q. What are the units or denomination for measuring market value and volume in the report?
A. The market size/industry size or the market value is measured in terms of USD Billion, while the market volume is measured in terms of units sold.
Q. What would be the CAGR of the Global Electronic Trial Master Files Market during 2020 to 2025?
A. The market size of Global Electronic Trial Master Files Market would register a CAGR of 12% during 2020-25.
Q. Which of the regions would be a bright spot for Global Electronic Trial Master Files Market during 2020-2025?
A. North America and Asia-Pacific are emerging as lucrative market with the improving healthcare infrastructure. Moreover, China and India are witnessing an upsurge with the increasing establishment of pharmaceutical companies and sale of good quality medicines at lower prices.
Q. Who are the key players or major competitors in Global Electronic Trial Master Files Market?
A. Oracle Corporation, Veeva Systems, Mastercontrol, Transperfect, Sureclinical, Lab Corp, Mayo Clinic, BIOVIA Corp., Aurea Software, Wingspan Technology. are few of the leading players with majority market share in Global Electronic Trial Master Files Market.
Q. Does the report scope cover Electronic Trial Master Files type wise market statistics also?
A. Yes, the scope of the report encompasses detailed Electronic Trial Master Files type wise statistics such as market size, market segmentation based on various subcategories.
Q. Which delivery segment would emerge as an opportunity area for players in Global Electronic Trial Master Files Market?
A. Cloud based segment grabbed the largest market share owing to the increasing adoption of cloud based solutions worldwide. Cloud based solutions offers flexibility, and affordability is expected to further driving the market of eTMF systems during 2020-25.
Q. Which End User segment would emerge as an opportunity area for players in Global Electronic Trial Master Files Market?
A. Mushrooming clinical trials and huge research and development budgets by biotechnology and pharmaceutical companies the demand for electronic trail master file systems market is witnessing a growth and is anticipated to grow during 2020-25.
Frequently Asked Questions
1. Introduction
1.1. Product Definition
1.2. Research Process
1.3. Assumptions
1.4. Market Segmentation
2. Executive Summary
3. Expert Verbatim- What our Experts Say?
4. Global Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
4.1. Market Size & Analysis
4.1.1. Market Revenue
4.2. Market Share & Analysis
4.2.1. By Component
4.2.1.1. Services
4.2.1.2. Software
4.2.2. By Mode of Delivery
4.2.2.1. Cloud-Based
4.2.2.2. On-Premise
4.2.3. By End User
4.2.3.1. Pharmaceutical and Biotechnology Companies
4.2.3.2. Contract Research Firms
4.2.4. By Region
4.2.4.1. North America
4.2.4.2. South America
4.2.4.3. Europe
4.2.4.4. Middle East and Africa
4.2.4.5. Asia-Pacific
4.2.5. By Company
4.2.5.1. Revenue Shares
4.2.5.2. Strategic Factorial Indexing
4.2.5.3. Competitor Placement in MarkNtel Quadrant
5. North America Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
5.1. Market Size & Analysis
5.1.1. Market Revenue
5.2. Market Share & Analysis
5.2.1. By Component
5.2.2. By Mode of Delivery
5.2.3. By End User
5.2.4. By Country
5.2.4.1. United States
5.2.4.2. Canada
5.3. United States Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
5.3.1. Market Size & Analysis
5.3.1.1. Market Revenue
5.3.2. Market Share & Analysis
5.3.2.1. By Component
5.3.2.2. By Mode of Delivery
5.3.2.3. By End User
5.4. Canada Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
5.4.1. Market Size & Analysis
5.4.1.1. Market Revenue
5.4.2. Market Share & Analysis
5.4.2.1. By Component
5.4.2.2. By Mode of Delivery
5.4.2.3. By End User
6. Latin America Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
6.1. Market Size & Analysis
6.1.1. Market Revenue
6.2. Market Share & Analysis
6.2.1. By Component
6.2.2. By Mode of Delivery
6.2.3. By End User
6.2.4. By Country
6.2.4.1. Brazil
6.2.4.2. Mexico
6.3. Brazil Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
6.3.1. Market Size & Analysis
6.3.1.1. Market Revenue
6.3.2. Market Share & Analysis
6.3.2.1. By Component
6.3.2.2. By Mode of Delivery
6.3.2.3. By End User
6.4. Mexico Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
6.4.1. Market Size & Analysis
6.4.1.1. Market Revenue
6.4.2. Market Share & Analysis
6.4.2.1. By Component
6.4.2.2. By Mode of Delivery
6.4.2.3. By End User
7. Europe Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
7.1. Market Size & Analysis
7.1.1. Market Revenue
7.2. Market Share & Analysis
7.2.1. By Component
7.2.2. By Mode of Delivery
7.2.3. By End User
7.2.4. By Country
7.2.4.1. France
7.2.4.2. Germany
7.2.4.3. United Kingdom
7.2.4.4. Spain
7.3. France Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
7.3.1. Market Size & Analysis
7.3.1.1. Market Revenue
7.3.2. Market Share & Analysis
7.3.2.1. By Component
7.3.2.2. By Mode of Delivery
7.3.2.3. By End User
7.4. Germany Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
7.4.1. Market Size & Analysis
7.4.1.1. Market Revenue
7.4.2. Market Share & Analysis
7.4.2.1. By Component
7.4.2.2. By Mode of Delivery
7.4.2.3. By End User
7.5. United Kingdom Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
7.5.1. Market Size & Analysis
7.5.1.1. Market Revenue
7.5.2. Market Share & Analysis
7.5.2.1. By Component
7.5.2.2. By Mode of Delivery
7.5.2.3. By End User
7.6. Spain Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
7.6.1. Market Size & Analysis
7.6.1.1. Market Revenue
7.6.2. Market Share & Analysis
7.6.2.1. By Component
7.6.2.2. By Mode of Delivery
7.6.2.3. By End User
8. Middle East and Africa Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
8.1. Market Size & Analysis
8.1.1. Market Revenue
8.2. Market Share & Analysis
8.2.1. By Component
8.2.2. By Mode of Delivery
8.2.3. By End User
8.2.4. By Country
8.2.4.1. Saudi Arabia
8.2.4.2. UAE
8.2.4.3. Qatar
8.3. GCC Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
8.3.1. Market Size & Analysis
8.3.1.1. Market Revenue
8.3.2. Market Share & Analysis
8.3.2.1. By Component
8.3.2.2. By Mode of Delivery
8.3.2.3. By End User
8.4. South Africa Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
8.4.1. Market Size & Analysis
8.4.1.1. Market Revenue
8.4.2. Market Share & Analysis
8.4.2.1. By Component
8.4.2.2. By Mode of Delivery
8.4.2.3. By End User
9. Asia-Pacific Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
9.1. Market Size & Analysis
9.1.1. Market Revenue
9.2. Market Share & Analysis
9.2.1. By Component
9.2.2. By Mode of Delivery
9.2.3. By End User
9.2.4. By Country
9.2.4.1. China
9.2.4.2. India
9.2.4.3. Japan
9.2.4.4. Australia
9.3. China Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
9.3.1. Market Size & Analysis
9.3.1.1. Market Revenue
9.3.2. Market Share & Analysis
9.3.2.1. By Component
9.3.2.2. By Mode of Delivery
9.3.2.3. By End User
9.4. India Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
9.4.1. Market Size & Analysis
9.4.1.1. Market Revenue
9.4.2. Market Share & Analysis
9.4.2.1. By Component
9.4.2.2. By Mode of Delivery
9.4.2.3. By End User
9.5. Japan Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
9.5.1. Market Size & Analysis
9.5.1.1. Market Revenue
9.5.2. Market Share & Analysis
9.5.2.1. By Component
9.5.2.2. By Mode of Delivery
9.5.2.3. By End User
9.6. Australia Electronic Trial Master File (eTMF) Systems Industry Market Analysis, 2015-2025F
9.6.1. Market Size & Analysis
9.6.1.1. Market Revenue
9.6.2. Market Share & Analysis
9.6.2.1. By Component
9.6.2.2. By Mode of Delivery
9.6.2.3. By End User
10. Global Electronic Trial Master File (eTMF) Systems Market Policies, Regulations, Product Standards
11. Global Electronic Trial Master File (eTMF) Systems Market Trends & Insights
12. Global Electronic Trial Master File (eTMF) Systems Market Dynamics
12.1.Growth Drivers
12.2.Challenges
12.3.Impact Analysis
13. Global Electronic Trial Master File (eTMF) Systems Market Hotspot & Opportunities
14. Global Electronic Trial Master File (eTMF) Systems Market Key Strategic Imperatives for Success & Growth
15. Global Competition Outlook
15.1. Competition Matrix
15.1.1. Product Portfolio
15.1.2. Brand Specialization
15.1.3. Target Markets
15.1.4. Target Mode of Delivery’s
15.1.5. Research & Development
15.1.6. Strategic Alliances
15.1.7. Strategic Initiatives
15.2. Company Profiles (Business Description, Product Segments, Business Segments, Financials, Strategic Alliances/ Partnerships, Future Plans)
15.2.1. Oracle Corporation
15.2.2. Veeva Systems
15.2.3. Mastercontrol
15.2.4. Transperfect
15.2.5. Sureclinical
15.2.6. Lab Corp
15.2.7. Mayo Clinic
15.2.8. BIOVIA Corp.
15.2.9. Aurea Software
15.2.10. Wingspan Technology
16. Disclaimer
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
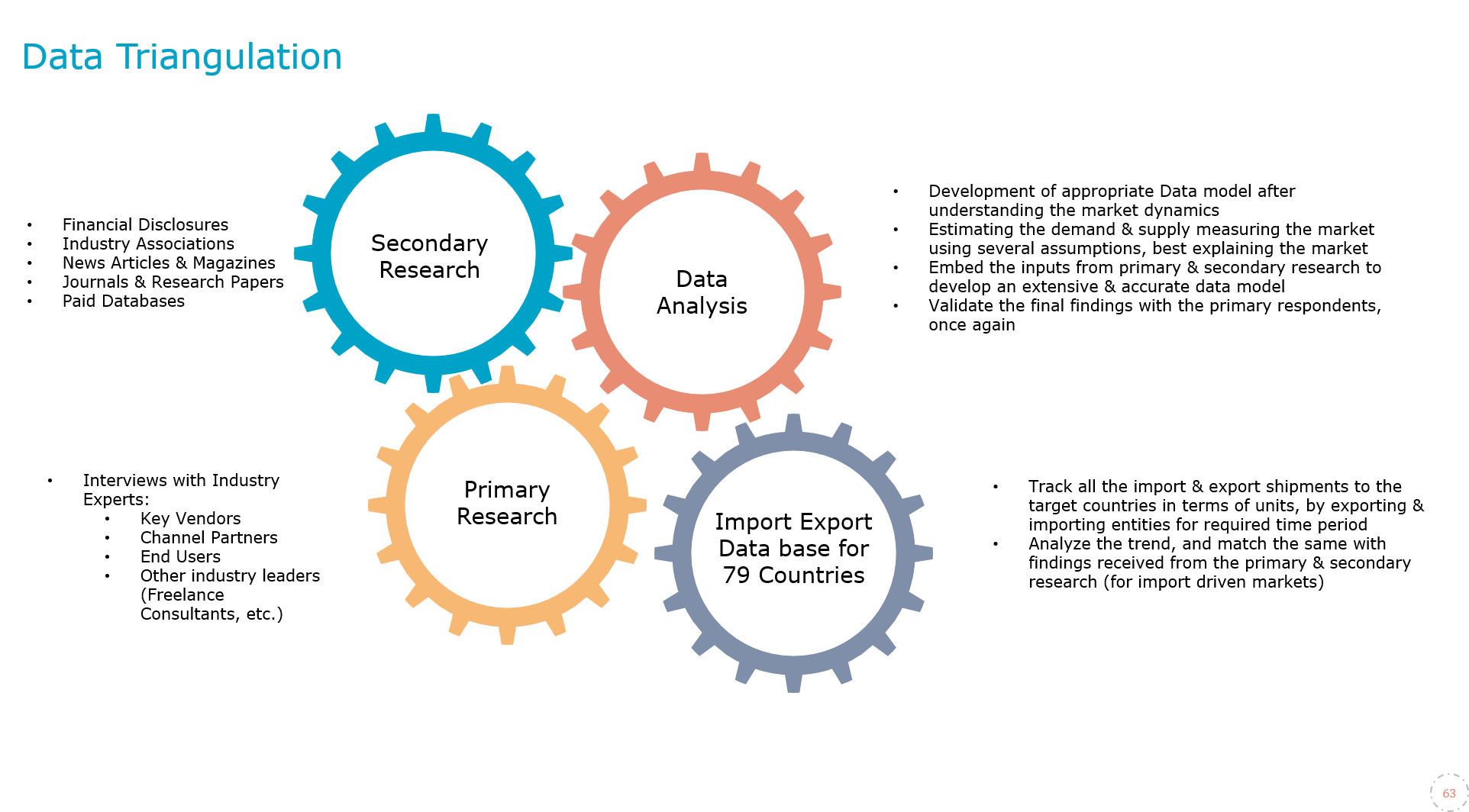
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making









