
By Drug Class (Yescarta (axicabtagene ciloleucel), Kymriah (tisagenlecleucel),Tecartus (brexucabtagene autoleucel), Breyanzi (lisocabtagene maraleucel), Others (Abecma (Idecabtagene vicleucel), etc.))... ... e vicleucel), etc.)), By Application (Acute Lymphocytic Leukemia (ALL), Diffuse Large B-cell Lymphoma (DLBCL), Follicular Lymphoma, Multiple Myeloma, Others (Mantle Cell Lymphoma, etc.)), By End-User (Hospitals, Clinics, Specialty Cancer Centers), By Region (North America, South America, Europe, Middle East & Africa, Asia-Pacific), By Country (The US, Canada, Mexico, Brazil, The UK, Germany, France, Italy, Spain, South Africa, GCC, China, Japan, India, Australia, South East Asia), By Company (Amgen Inc., Bluebird Bio, Inc., Bristol Myers Squibb, CARsgen Therapeutics, Gilead Sciences, Inc., Merck & Co., Inc., Novartis International AG, Pfizer Inc., Sorrento Therapeutics, Inc., Others) Read more
- Healthcare
- Jun 2022
- 192
- PDF, Excel, PPT
Market Definition
CAR T-cell therapy is a type of immunotherapy that uses altered immune cells (T-cells) to find & attack tumor cells. During the therapy, a patient’s T cells are extracted & genetically altered to express a CAR (Chimeric Antigen Receptor) that recognizes a protein on the surface of cancer cells. The modified T-cells are then multiplied & infused back into the patient, where they identify & destroy tumor cells and might remain in the body for months after the infusion.
The therapy can result in severe side effects, which is why patients stay in the hospital for a week or two for monitoring. Its efficacy in the treatment of hematologic malignancies has been well-proved, and there are numerous ongoing research activities to identify if it helps treat solid tumors.
Market Insights
The Global Chimeric Antigen Receptor (CAR) T-cell Therapy Market is projected to register exponential CAGR during the forecast period, i.e., 2022-27. The growth of the market would be driven primarily by the burgeoning adoption of CAR T-cell therapy to address the rising patient pool for blood cancers, including lymphomas, certain forms of leukemia, & also multiple myeloma, on account of the mounting awareness regarding its benefits over traditional treatments.
| Report Coverage | Details |
|---|---|
| Study Period | Historical Data: 2017-20 |
| Base Year: 2021 | |
| Forecast Period: 2022-27 | |
| Regions Covered | North America: The US, Canada, Mexico |
| Europe: Germany, The UK, Germany, France, Italy, Spain, Rest of Europe | |
| Asia-Pacific: China, Japan, India, Australia, South East Asia, Rest of Asia-Pacific | |
| South America: Brazil, Rest of Latin America | |
| Middle East & Africa: South Africa, GCC, Rest of Middle East & Africa | |
| Key Companies Profiled |
Amgen Inc., Bluebird Bio, Inc., Bristol Myers Squibb, CARsgen Therapeutics, Gilead Sciences, Inc., Merck & Co., Inc., Novartis International AG, Pfizer Inc., Sorrento Therapeutics, Inc., Others |
| Unit Denominations | USD Million/Billion |
Various ongoing R&D activities in the life science & biotechnology sectors across numerous countries for blood cancer treatment using chimeric antigen receptors to evaluate CAR T-cell therapy's effectiveness, safety, mechanism of drug action, and adherence in patients with lymphoma & leukemia are also augmenting the overall growth of the global market.
The approvals of Kymriah & Yescarta earlier in 2017, their expansion across Japan & Europe during 2018-19, and their steadily growing global sales have proved to be a milestone for the Global CAR T-cell Therapy Market. Since then, there have been rapid & notable developments in the field as researchers are tweaking the efficacy & safety of CAR T-cells using novel approaches.
Despite the immense success of CAR T-cell therapy in treating blood cancers, there's still a major missing to its full potential since its application is limited only to certain liquid tumors in relapsed & refractory stages. Challenges like complex manufacturing & supply chain, high costs, and delayed preapproval reimbursement for patients are affecting its commercialization and, in turn, hampering the market growth.
However, with the optimization of autologous CAR T-cells for liquid malignancies, expansion of healthcare facilities, and fast & innovative developments of effective biomarkers to treat solid tumors, the Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market could witness remunerative prospects over the coming years.
- Introduction
- Research Process
- Assumption
- Market Segmentation
- Market Definition
- Executive Summary
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Trends & Insights
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Regulation & Policy, By Country
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Dynamics
- Growth Drivers
- Challenges
- Impact Analysis
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Hotspot and Opportunities
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- Yescarta (axicabtagene ciloleucel)
- Kymriah (tisagenlecleucel)
- Tecartus (brexucabtagene autoleucel)
- Breyanzi (lisocabtagene maraleucel)
- Others (Abecma (Idecabtagene vicleucel), etc.)
- By Application
- Acute Lymphocytic Leukemia (ALL)
- Diffuse Large B-cell Lymphoma (DLBCL)
- Follicular Lymphoma
- Multiple Myeloma
- Others (Mantle Cell Lymphoma, etc.)
- By End-User
- Hospitals
- Clinics
- Specialty Cancer Centers
- By Region
- North America
- South America
- Europe
- Middle East & Africa
- Asia-Pacific
- By Company
- Competition Characteristics
- Revenue Shares
- Competitor Placement in MarkNtel Advisor’s Quadrant
- By Drug Class
- Market Size and Analysis
- North America Chimeric Antigen Receptor (CAR) T-Cell Therapy Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- By Application
- By End-User
- By Country
- The US
- Canada
- Mexico
- Market Size and Analysis
- South America Chimeric Antigen Receptor (CAR) T-Cell Therapy Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- By Application
- By End-User
- By Country
- Brazil
- Rest of Latin America
- Market Size and Analysis
- Europe Chimeric Antigen Receptor (CAR) T-Cell Therapy Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- By Application
- By End-User
- By Country
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Market Size and Analysis
- Middle East & Africa Chimeric Antigen Receptor (CAR) T-Cell Therapy Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- By Application
- By End-User
- By Country
- South Africa
- GCC
- Rest of Middle East & Africa
- Market Size and Analysis
- Asia-Pacific Chimeric Antigen Receptor (CAR) T-Cell Therapy Outlook, 2017- 2027
- Market Size and Analysis
- By Revenues in USD Million
- Market Share and Analysis
- By Drug Class
- By Application
- By End-User
- By Country
- China
- Japan
- India
- Australia
- South East Asia
- Rest of Asia Pacific
- Market Size and Analysis
- Global Chimeric Antigen Receptor (CAR) T-Cell Therapy Market Key Strategic Imperatives for Success and Growth
- Competitive Outlook
- Competition Matrix
- By Application Portfolio
- Brand Specialization
- Target Markets
- Target by Applications
- Research & Development
- Strategic Alliances
- Strategic Initiatives
- Company Profiles (Business Description, By Application Segments, Business Segments, Financials, Strategic Alliances/ Partnerships, Future Plans)
- Amgen Inc.
- Bluebird Bio, Inc.
- Bristol Myers Squibb
- CARsgen Therapeutics
- Gilead Sciences, Inc.
- Merck & Co., Inc.
- Novartis International AG
- Pfizer Inc.
- Sorrento Therapeutics, Inc.
- Others
- Competition Matrix
- Disclaimer
_T-Cell_Therapy_Market_-Segmentation.png)
MarkNtel Advisors follows a robust and iterative research methodology designed to ensure maximum accuracy and minimize deviation in market estimates and forecasts. Our approach combines both bottom-up and top-down techniques to effectively segment and quantify various aspects of the market. A consistent feature across all our research reports is data triangulation, which examines the market from three distinct perspectives to validate findings. Key components of our research process include:
1. Scope & Research Design At the outset, MarkNtel Advisors define the research objectives and formulate pertinent questions. This phase involves determining the type of research—qualitative or quantitative—and designing a methodology that outlines data collection methods, target demographics, and analytical tools. They also establish timelines and budgets to ensure the research aligns with client goals.
2. Sample Selection and Data Collection In this stage, the firm identifies the target audience and determines the appropriate sample size to ensure representativeness. They employ various sampling methods, such as random or stratified sampling, based on the research objectives. Data collection is carried out using tools like surveys, interviews, and observations, ensuring the gathered data is reliable and relevant.
3. Data Analysis and Validation Once data is collected, MarkNtel Advisors undertake a rigorous analysis process. This includes cleaning the data to remove inconsistencies, employing statistical software for quantitative analysis, and thematic analysis for qualitative data. Validation steps are taken to ensure the accuracy and reliability of the findings, minimizing biases and errors.
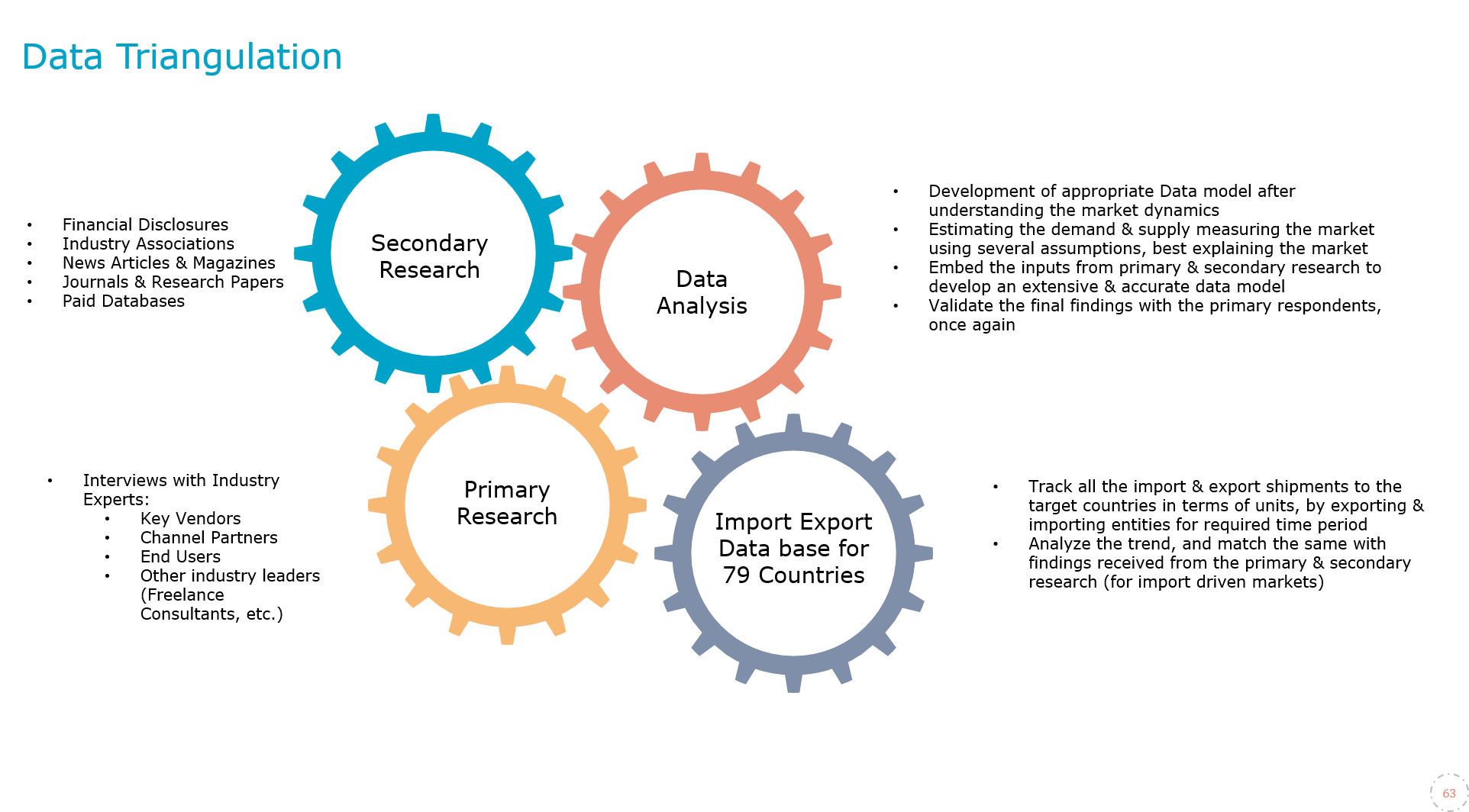
4. Data Forecast and FinalizationThe final phase involves forecasting future market trends based on the analyzed data. MarkNtel Advisors utilize predictive modeling and time series analysis to anticipate market behaviors. The insights are then compiled into comprehensive reports, featuring visual aids like charts and graphs, and include strategic recommendations to inform client decision-making
